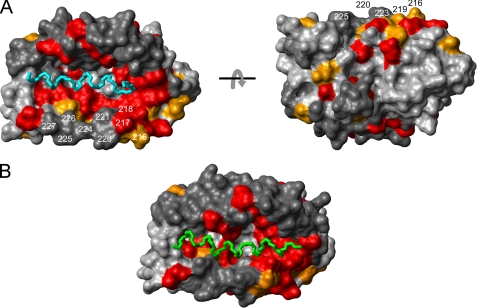
FIGURE 6.
The α-helices of the KIS domain and the Kv4.3 N terminus can bind to the same surface pocket on the KChIP core structure. A, mapping of the NMR chemical shift perturbation data (Fig. 4A) onto a surface representation of KChIP1 in the KChIP1-Kv4.3 N terminus x-ray structure (14) (PDB entry 2I2R). Color coding and residue numbers in the KChIP4a numbering system are as in Fig. 4B. The two views are related by a 180° rotation around the x-axis. The main chain of the α-helical segment of the Kv4.3 N terminus buried in the hydrophobic groove of KChIP1 is shown in cyan (Ala3–Met20). B, chemical shift-driven docking of the KIS domain α-helix (green) to the same KChIP1 structure as in A. The docking was performed with HADDOCK 1.3 (32).