Figure 5.
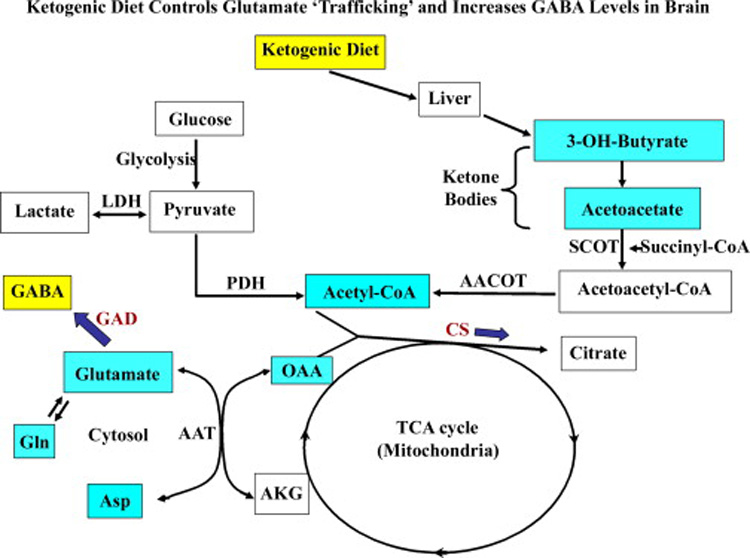
A schematic representation of the metabolism of glucose, ketone bodies and amino acids (excitatory neurotransmitters) in the brain. Approximately 90% of dietary calories derived from fats induce ketosis through fatty acid metabolism in the liver. The major ketone bodies produced by this route comprise 3-hydroxy-butyrate (3-OH-butyrate) and acetoacetate, which serve as fuel for high-energy demand of the brain in epileptic conditions. The ketone body acetoacetate is sequestered to acetoacetyl-CoA by succinyl-CoA transferase (SCOT) in the brain. In parallel, the pyruvate (through glycolysis) generates acetyl-CoA by pyruvate dehydrogenase complex (PDH) and a fraction of lactate through lactate dehydrogenase (LDH). The acetoacetyl-CoA routed via ketone bodies metabolism also generates excess pool of acetyl-CoA in the mitochondrial acetoacetyl-CoA thiolase (AACOT) reaction and enters the tricarboxylic acid (TCA) cycle. Hence, oxaloacetate (OAA) pool diminishes because of increased availability of substrates for key TCA cycle enzyme citrate synthetase (CS). As a result, less OAA is available for transamination reaction of aspartate aminotransferase (AAT) to produce aspartate, which in turn leads to increased glutamate pool for glutamic acid decarboxylase (GAD) and favors GABA synthesis. Alternatively, glutamine (Gln) production would likely reduce the glutamate load in neurons.
