Fig. 1.
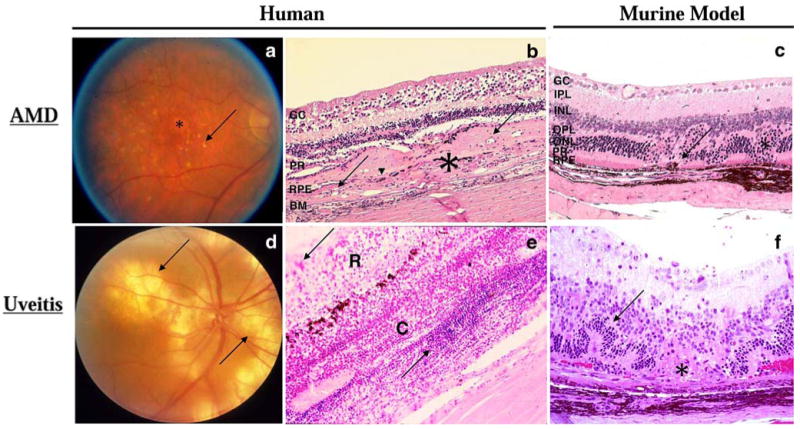
Fundoscopic and histologic representations of AMD and uveitis. a Fundoscopy of a human eye with AMD. Many drusen (arrow) and regions of RPE atrophy (asterisk) are visible by fundoscopy in a patient with dry AMD. b Photomicrograph of a human retina with wet AMD. Photoreceptor cells and most RPE (arrowhead) are replaced by a thick layer of fibrovascular tissue (asterisk) including small neovascular lumens (arrows). Ganglion cell layer, GC; photoreceptor layer, PR; RPE layer, RPE; and Bruch membrane, BM. (H & E, original magnification ×100). c Photomicrograph of a Cx3cr1−/−/Ccl2−/− mouse retina, a model for AMD. Choroidal neovascularization (arrows) and photoreceptor lesions (asterisk) are observed. Ganglion cell layer, GC; inner plexiform layer, IPL; inner nuclear layer, INL; outer plexiform layer, OPL; outer nuclear layer, ONL; photoreceptor layer, PR; and RPE layer, RPE. (H & E, original magnification ×200). d Fundoscopy of a human uveitic eye. Multiple large subretinal infiltrates (arrows) are visible by fundoscopy in a patient with Vogt–Koyanagi–Harada syndrome. e Photomicrograph of a human retina with uveitis. Massive inflammatory cellular infiltration (arrows) is seen in the edematous retina (R) and a thickened choroid (C) of a patient with sympathetic ophthalmia. (H & E, original magnification ×100). f Photomicrograph of a B10A mouse retina with experimental autoimmune uveitis. Focal retinal outer layer destruction (asterisk), retinal folds (arrow), and retinal and choroidal inflammatory cells are visible. (H & E, original magnification ×200)
