Fig. 5. Mapping of the interaction domain on Pyst1.
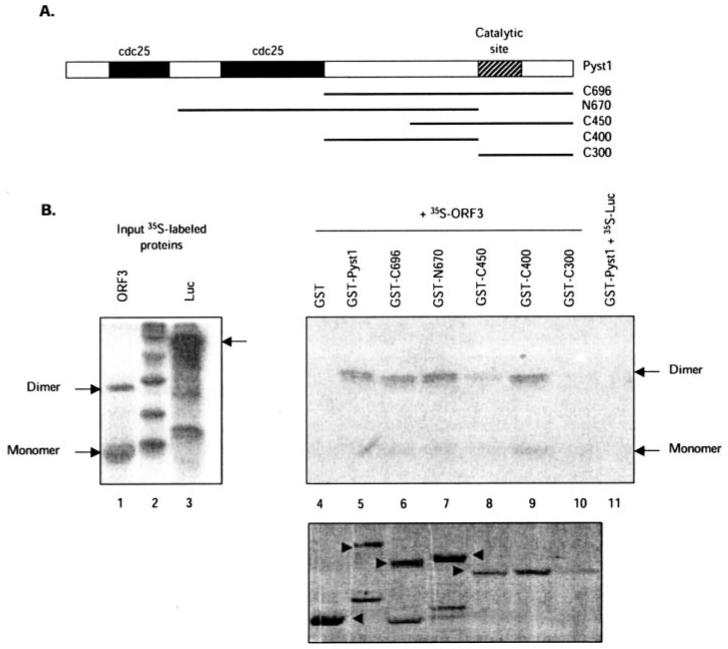
A, the Pyst1 protein and its mutants used in this study are illustrated. The two N-terminal Cdc25 homology domains and the C-terminal catalytic site are shown. B, GST pull-down assay for pORF3-Pyst1 interaction. The ORF3 protein (lane 1) or a control luciferase protein (lane 3) were expressed and labeled with [35S]methionine-cysteine in a coupled in vitro transcription-translation reaction. The labeled proteins were then incubated with glutathione-Sepharose beads containing equivalent amounts of GST-fused full-length (lane 5) or mutant Pyst1 (lanes 6-10) proteins, and the retained pORF3 was analyzed as described under “Experimental Procedures.” As controls, pORF3 retained on GST beads (lane 4) or 35S-labeled luciferase (Luc) retained on GST-Pyst1 beads (lane 11) was also analyzed. The pORF3 monomer and dimer are indicated. The bottom panel shows GST and GST-Pyst1 fusion proteins (arrowheads) used for the pull-down.
