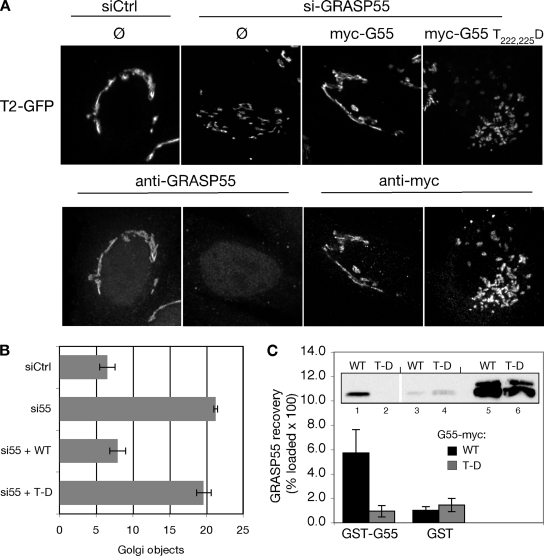
Figure 8.
Aspartic acid substitution at threonines 222 and 225 inhibits Golgi linking and oligomerization activities of GRASP55. (A) Golgi ribbon integrity was assessed in control (siCtrl) or GRASP55 (siGRASP55) knockdown cells without gene replacement (Ø) or after replacement with myc-tagged GRASP55 or GRASP55 T222,225D. Images show GalNAcT2-GFP fluorescence (top) and in the same cells either anti-GRASP55 staining or anti-myc staining (bottom). Bar, 4 μm. (B) The number of Golgi objects per cell was counted using the automated protocol described in the Figure 1 legend (n = 4, ±SEM). (C) Extracts from HeLa cells transfected with either wild-type GRASP55 (WT) or GRASP55 T222,225D (T-D) were incubated with GST-GRASP55 (lanes 1 and 2) or GST alone (lanes 3 and 4) immobilized on glutathione-agarose beads. Recovery was determined by immunoblotting with anti-myc antibodies. Values shown represent the percentage of loaded protein (lanes 5–6) bound to beads × 100 (n = 5, ±SEM).