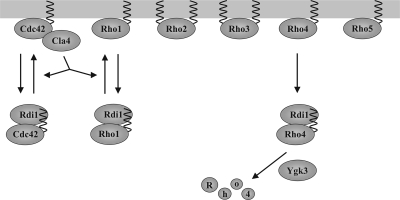
Figure 10.
Model for the regulation of Rho GTPases by Rdi1. Rdi1 selectively extracts Cdc42, Rho1, and Rho4 from membranes and forms a complex with these proteins. Rho2 and Rho3 not only attach to the membrane through a prenyl group but also are palmitoylated. Cla4 disrupts binding between Rdi1 and Cdc42 and between Rdi1 and Rho1. Because Cla4 acts as a downstream effector of Cdc42, both proteins may constitute a positive feedback loop involved in the establishment of cell polarity. Furthermore, Cdc42 may regulate Rho1 activity via Cla4. The GSK-3β homologue Ygk3 and Rdi1 target Rho4 for degradation by vacuolar proteases and the proteasome after membrane extraction.