Figure 1.
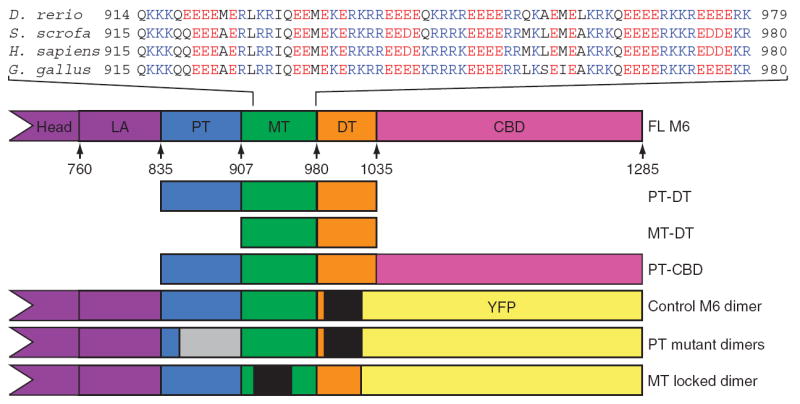
M6 tail domains and experimental constructs. The tail domains of M6 are indicated in the context of the full-length protein, with the position of the first residue of each domain in the human sequence annotated. The calmodulin binding domains are the heretofore known elements of the lever arm (LA); the end of the IQ helix is residue 835. Sequences from the MT from four species are presented to show the repeating-charge pattern, which switches approximately every four residues. The E. coli – expressed tail fragments are shown along with the construct name. The control M6 dimer, the MT locked mutant and the PT mutant constructs were modified by insertion of a GCN4 segment (black regions) to ensure dimerization at the low concentrations used for single-molecule analyses and by replacing the cargo binding domain (CBD) with YFP to provide a specific surface-attachment point via a YFP monoclonal antibody. The location of the randomized PT is gray.
