Figure 3.
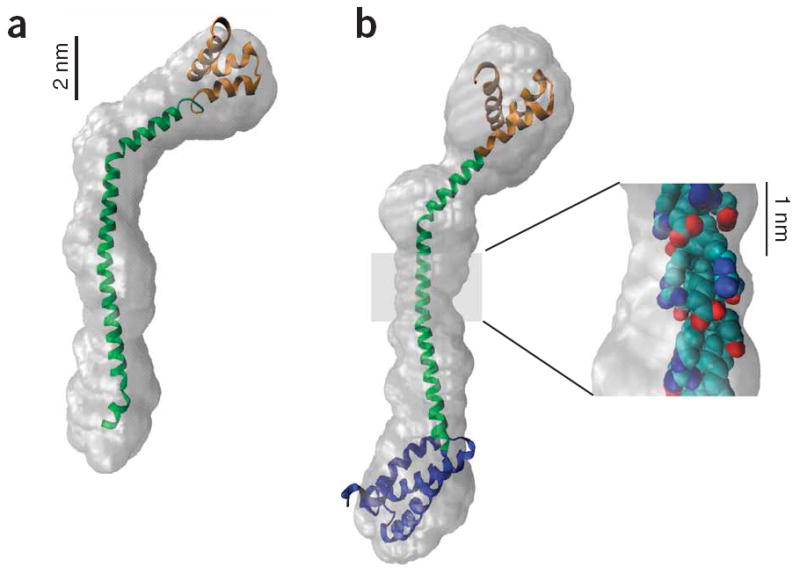
SAXS envelope reconstructions of tail domains. (a) A model of the MT-DT structure with the MT structure (green) derived from the single α-helix prediction and the DT structure (orange) from a Rosetta prediction28. The model was constructed by aligning the peptide backbone manually to the consensus best GASBOR reconstruction and then docking the model into the filtered GASBOR reconstruction envelope using the Situs software package51. (b) A model of the PT-DT structure was constructed by adding a Rosetta prediction for the PT structure (blue) to the N terminus of the MT-DT model in a. This model was docked as above into the filtered GASBOR reconstruction. Note that even though the number of residues has increased from 129 to 201 (56%), the envelope is only ~3 nm longer, indicating a compact PT. Inset, the structure of a segment of the highly charged MT (residues 935–955), with the side chain atoms color coded by charge, revealing bands of charge circling the helix and providing stabilizing i to i+4 charge-charge interactions.
