Figure 6.
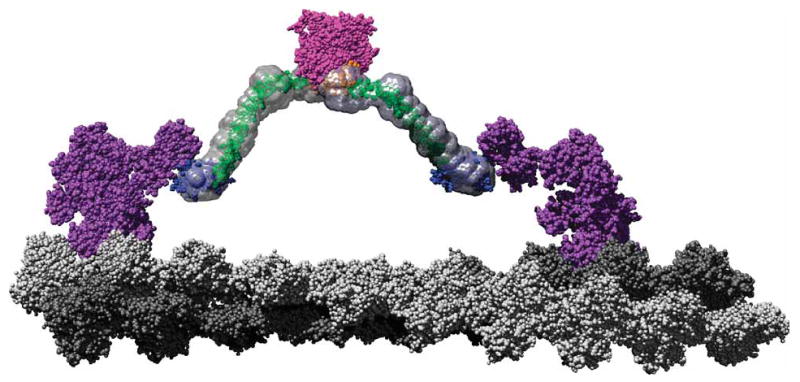
A scale model of a M6 dimer moving along an actin filament. The F-actin–docked myosin model is based on that proposed by Holmes et al.54 Monomers corresponding to the two protofilaments are colored in light and dark gray, respectively, to emphasize the pseudorepeat at 36 nm. Using a structural alignment in PyMOL (http://pymol.sourceforge.net), the post-stroke structure of the M6 head (PDB 2BKI5) was docked onto the filament. The prestroke structure (PDB 2V2619) was also docked onto the actin filament 13 monomers removed from the post-stroke head. These structures along with the associated light chains are shown in purple. The tail model presented in Figure 3b, with the same color scheme, has been fused to the end of the IQ domains such that the PT projects along the same vector as the IQ helix (cyan) and then rotates around the Gly839 to remove steric clashes. This represents an orientation where the PT maximally contributes to the M6 stroke, which is one of many potential angles at which it meets the IQ domain. The cargo binding domains are shown in close association, as their dimerization suggests. The SAXS envelopes were then superimposed on the model to place the data in context of a working motor using Chimera55. This model shows that the proposed roles for the tail domains are clearly compatible with a 36-nm processive step for a M6 dimer.
