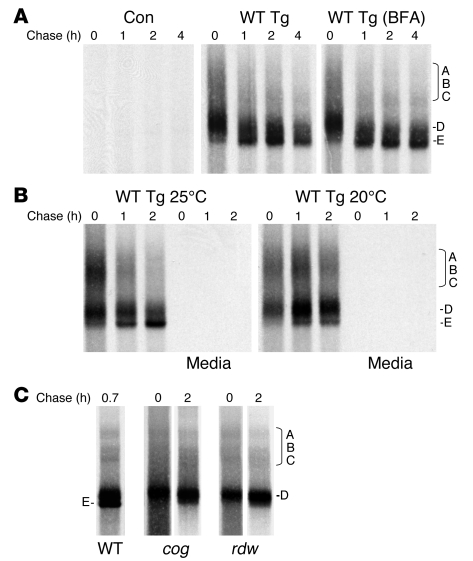
Figure 2. Arrested disulfide maturation of Tg bearing a mutation in the ChEL domain.
(A) 293 cells were transiently transfected with vector DNA encoding wild-type Tg (bearing a C-terminal myc-6xHis epitope tag that does not block Tg secretion; ref. 37) or empty vector (control [Con]). Transfected cells were then pulse labeled for 30 minutes with 35S-labeled amino acids and chased at 37°C for up to 4 hours in the absence or presence of BFA (5 μg/ml) where indicated. At each chase time, cells were lysed, immunoprecipitated with anti-Tg, and analyzed by nonreducing 4% SDS-PAGE and fluorography. A region of the gel enriched in disulfide-linked Tg adducts A, B, and C (28) is indicated. Two additional forms of Tg monomer — folding intermediate D and the E band representing fully oxidized mature Tg — are shown. (B) Results of an experiment identical to the one represented in A, except that the cells were chased at either 25°C (left) or 20°C (right). At each time, chase medium was also collected (as indicated), but at these temperatures the media contained no labeled Tg. (C) Results of an experiment identical to the one represented in A, except that cells were transfected with plasmid DNA encoding cog (L2263P) or rdw (G2300R) mutations within the Tg ChEL domain, and the cell lysis buffer included 20 mM N-ethylmaleimide. No detectable Tg was secreted, so analysis of the supernatant is not shown.