Abstract
The goal of this study was to examine behavioral and electrophysiological correlates of involuntary orienting toward rapidly presented angry faces in non-anxious, healthy adults using a dot-probe task in conjunction with high-density event-related potentials and a distributed source localization technique. Consistent with previous studies, participants showed hypervigilance toward angry faces, as indexed by facilitated response time for validly cued probes following angry faces and an enhanced P1 component. An opposite pattern was found for happy faces suggesting that attention was directed toward the relatively more threatening stimuli within the visual field (neutral faces). Source localization of the P1 effect for angry faces indicated increased activity within the anterior cingulate cortex, possibly reflecting conflict experienced during invalidly cued trials. No modulation of the early C1 component was found for affect or spatial attention. Furthermore, the face-sensitive N170 was not modulated by emotional expression. Results suggest that the earliest modulation of spatial attention by face stimuli is manifested in the P1 component, and provide insights about mechanisms underlying attentional orienting toward cues of threat and social disapproval.
Keywords: spatial attention, anger, face perception, event-related potentials, source localization
Electrophysiological correlates of spatial orienting towards angry faces: A source localization study
Perception of the human face, as well as the social cues derived from it, is central to social interaction and in the communication of threat (Argyle, 1983), and occurs rapidly, within 100 ms of presentation (e.g., Liu, Harris, & Kanwisher, 2002). For healthy individuals, visual scanpaths of the human face are directed to salient features that define facial emotional expressions such as the mouth and eyes (Walker-Smith, Gale & Findlay, 1977; Mertens, Siegmund, & Grusser, 1993) and this tendency increases for emotional facial expression (Horley, Williams, Gonsalvez, & Gordon, 2004), especially as the identification of threat increases (Mogg & Bradley, 1998, 1999; Rohner, 2002). Angry faces, in particular, signal social disapproval and threat (Öhman, 1986), and the violation of social rules or expectations (Averill, 1983).
Given the biological and social significance of cues of social disapproval, it is not surprising that angry faces are detected more efficiently than friendly faces. In healthy populations, a processing bias toward threat-related (angry) faces has been demonstrated in visual search tasks using both schematic (Eastwood, Smilek, & Merikle, 2001; Fox, Lester, Russo, Bowles, Pichler, & Dutton, 2000) and real (Horstmann & Bauland, 2006; William, Moss, Bradshaw, & Mattingley, 2005) face stimuli. Of note, this bias is further potentiated in anxious populations (e.g., generalized anxiety disorder, social phobia) whose fear of negative evaluation and socially threatening situations makes angry faces more salient (Clark & Wells, 1995; Heinrichs & Hofmann, 2001; Kolassa, & Miltner, 2006; Mogg, Philippot, & Bradley, 2004).
Until recently, attentional bias toward angry and happy face pairs had only been observed for anxious populations using a visuospatial ‘dot-probe task’ (e.g., Bradley, Mogg, Fall, & Hamilton, 1998; Mogg, Philippot, & Bradley, 2004) but not for healthy individuals. In this task, two facial expressions, varying in affective valence (e.g., angry/ neutral, happy/neutral) are simultaneously presented to participants in the left or right visual field. Following presentation of the face pair (cue), a neutral bar probe (target requiring a response) appears in the location previously occupied by one of the faces. If attention is selectively directed toward threat, probes presented in the location of the angry face (valid cue trial) should be identified faster than probes presented in the location of neutral faces (the unattended stimulus; invalid cue trial). As such, the threat-related face stimulus acts as an exogenous spatial cue. Cooper and Langton (2006) recently demonstrated that, provided the face pair is presented very rapidly (i.e., 100 ms as opposed to the standard 500 ms presentation), threat-related stimuli do have a modulatory role on the control of spatial attention in healthy individuals. There was, however, a bias toward the neutral face in the happy/neutral face pair suggesting that attention was initially allocated to the most threatening (or least friendly) face (Mogg & Bradley, 1998, 1999; Rohner, 2002).
The dot-probe task has been criticized for being an indirect measure of covert orienting of attention, since inferences must be made from response time performance (e.g., Horley et al., 2004). One way to obtain a more direct, physiological measure of attention is to examine event-related brain potentials (ERPs) during this task. ERPs to both cue and probe stimuli may provide useful data on both the timing and neural substrates of threat-related attention bias. To date, ERP studies examining attentional bias have only used fearful faces in combination with happy faces (e.g., Bar-Haim, Lamy, & Glickman, 2005; Pourtois, Grandjean, Snader, & Vuilleumier, 2004).
Neuropsychological correlates of spatial orienting towards threat
The occipital P1 following cue and probe presentation and the superior parietal N1 following probe presentation provide an index of covert visuospatial orienting of attention (Hillyard, Luck, & Mangun, 1994). Enhancement of the P1 and N1 has been noted for attended stimuli and faster responses to probes (Eimer, 1994; Luck, Hillyard, Mouloua, Woldorff, Clark, & Hawkins, 1994). These findings are less consistent for the N1, as others have reported decreased N1 amplitudes or no effects (Pourtois et al., 2004) for validly cued attentional orienting (Fu, Fan, Chen, & Zhou, 2001; Fu, Caggiano, Greenwood, & Parasuraman, 2005).
Using a dot-probe task with fearful/neutral and happy/neutral face pairs, Pourtois and colleagues (Pourtois et al., 2004) reported that the P1 (but not the N1) was larger for valid trials compared with invalid trials for the fear/neutral face pair. Consistent with decreased response time to validly cued trials, results suggested that threat-related faces control the allocation of attention by involuntary orienting mechanisms. The P1 has been localized to extrastriate visual areas (lateral occipital and inferior temporal cortex). In a later ERP source localization study these authors noted that activity in the extrastriate cortex was enhanced for fear valid compared with fear invalid or happy conditions (Pourtois, Schwartz, Seghier, Lazeyras, & Vulleumier, 2006), possibly reflecting top-down influences from the fronto-parietal network or direct influences from limbic structures critically implicated in automatic responses to threat-related cures, such as the amygdala (Adolphs, Baron-Cohen, & Tranel, 2002). Interestingly, enhanced activity in the medial frontal/anterior cingulate cortex (ACC) in response to invalidly cued probes by fearful faces was also found, likely reflecting sensory or motor conflict and task difficulty (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Bush, Luu, & Posner, 2000).
An earlier component, the C1, has an onset latency of 50 ms following stimulus presentation, and is thought to reflect initial activity of the primary visual cortex. The C1, however, has not shown to be modulated by attention (e.g., Clark & Hillyard, 1995; Di Russo, Martinez, & Hillyard, 2003; Mangun, 1995; Martinez, Anllo-Vento, Sereno, Frank, Buxton, Bubowitz et al., 1999) indicating that early effects of spatial attention manifest later in the P1 and N1 (Di Russo et al., 2003). Interestingly, Pourtois et al. (2004) reported that enhanced C1 amplitudes in response to fearful faces were related to larger subsequent validity effects on the P1 (i.e., valid P1 – invalid P1). The results were exploratory, but support the claim that early (< 100 ms) neural activation arising from the primary visual cortex may be modulated by emotional valence (see also Halgren, Raij, Marinkovic, Jousmaeki, & Hari, 2000; Holmes, Vuilleumier, & Eimer, 2003; Pizzagalli, Regard & Lehmann, 1999; Pizzagalli, Lehmann, Hendrick, Regard, & Pasacual-Marqui, 2002).
Finally, the occipital temporal N170 is a face-specific component (unrelated to spatial attention) that may provide an index of rapid structural encoding of faces (e.g., Bentin, Allison, Puce, Perez & McCarthy, 1996). But whether or not the amplitude of the N170 is modulated by emotion is still a matter of debate (see for example Blau, Maurer, Tottenham, & McCandliss, 2007; Eimer & Holmes, 2007).
The present study
The purpose of the present study was to examine the involuntary orienting response towards emotional face cues in healthy individuals. To our knowledge, this is the first study investigating brain mechanisms underlying involuntary orienting using a dot-probe task with angry/neutral and happy/neutral face pairs in conjunction with high-density ERP recordings and distributed source localization techniques (see Pourtois et al., 2004 for a prior study using fearful faces and a similar approach). To examine initial orienting, a 100-ms face presentation time was used, as this time is likely too short to allow for shifts in gaze between stimuli. Similar to previous studies, we hypothesized that if attention was directed toward threat and modulate spatial orienting, response times would be faster for validly cued angry trials compared to (1) invalidly cued angry trials and (2) validly cued happy faces. Moreover, we hypothesized that enhanced spatial attention during validly cued angry trials would be manifested in increased P1 and N1 amplitudes time-locked to the probe. Based on prior findings with the dot-probe paradigm (Pourtois et al., 2004), the C1 and N170 time-locked to the face pair presentation were expected to be insensitive to emotional valence. Finally, Low Resolution Electromagnetic Tomography (LORETA; Pascual-Marqui et al., 1994) was used in conjunction with a high-density EEG array to investigate intracerebral sources underlying significant scalp ERP findings.
Method
Participants
Sixteen adults (M = 21.69 years, SD = 4.6 years; 8 men) were recruited from the Harvard University undergraduate psychology study pool. Participants were right-handed (Chapman & Chapman, 1987), reported no history or current unstable medical illness, head injury or neurological illness and were currently not taking any medications. Participants provided informed written consent and received course credit for their participation. The study was approved by the Committee on the Use of Human Subjects at Harvard University.
Dot-probe task
Stimuli
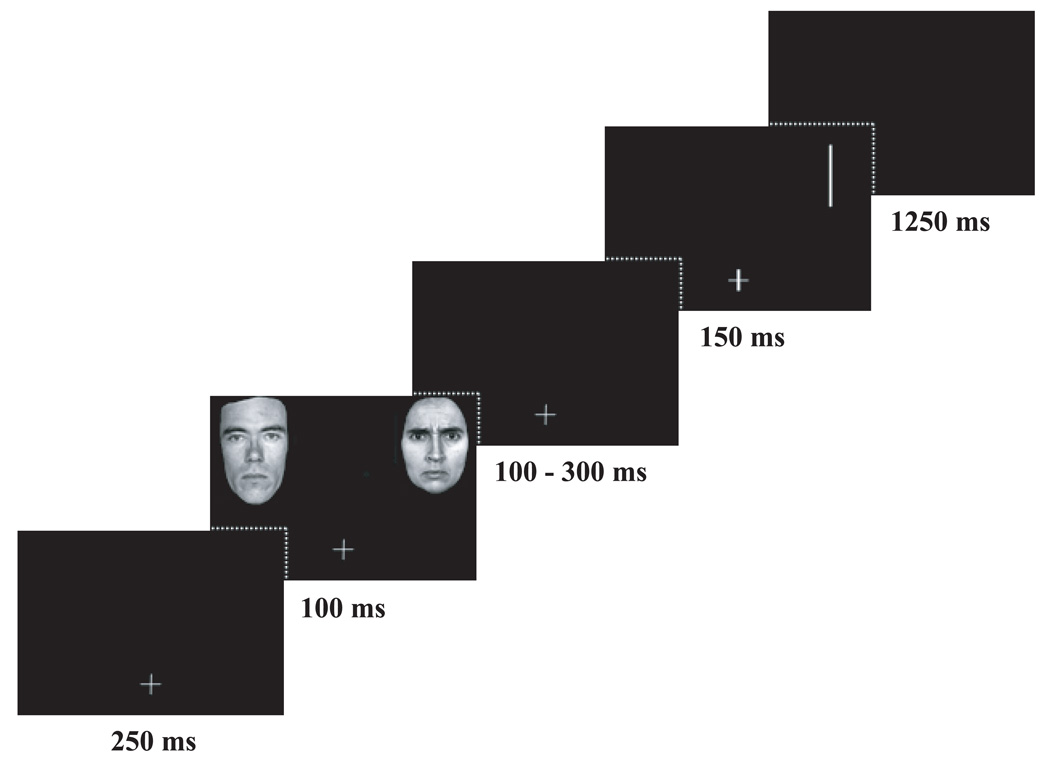
Pairs of face stimuli were created using gray-scale photographs of male and female identities portraying a neutral, happy or angry facial expression from the standardized Ekman series (Ekman & Friesen, 1976; see also Cooper & Langton, 2006; Pourtois et al., 2004 for design). Each face pair consisted of two different identities of the same sex portraying a neutral expression and either a happy or angry emotional expression yielding four conditions: neutral – angry, angry – neutral, neutral – happy, happy – neutral (Figure 1). Each emotion expression appeared equally often to the left or right of the neutral expression. Face stimuli were trimmed to exclude hair and non-facial contours and set on a black background. The faces measured 7.0 × 10.5 cm, subtending 6.5° visual angle (measured from participant to bottom inner corner of face stimulus) and viewed at a distance of 70 cm (a chin rest was used to maintain the distance between the participants and the screen constant). The faces were presented in the upper visual field with a distance of 4 cm between the horizontal median and outer edge and each face was equidistant from the vertical median. The probe was a white rectangular bar (horizontal or vertical) measuring 6.7 cm (0.3 cm thick) presented on either the left or right side of the screen in the same upper visual field location as the faces. The fixation cross measured 1.9 × 1.9 cm and was presented centrally in the lower part of the computer screen. All stimuli were set on a black background and presented on a 17 in. computer screen with a PC Pentium 3 running E-Prime.
Figure 1.
Schematic representation of the dot-probe paradigm.
Procedure
The dot-probe task began with one practice block of 16 trials followed by 9 blocks of 80 trials (total 720 trials). Each block was separated by a short rest break. Each trial began with the presentation of a fixation cross for 250 ms followed by a delay of 250 ms and then presentation of a face pair (the ‘cue’) for 100 ms. Interstimulus interval (ISI) varied randomly from 100–300 ms (in 50 ms increments), thus our stimulus onset asynchrony (SOA) was 200–400 ms. The probe then appeared for 150 ms in either location previously occupied by a face. The inter-trial interval was 1250 ms (Figure 1). Female face pairs were presented 60% of the time while male face pairs were presented 40% of the time. Happy and angry face pairs appeared equally often and with equal frequency in the right visual field (RVF) or left visual field (LVF). All stimuli were randomized and counterbalanced across participants.
As in Pourtois et al. (2004), a go/no-go paradigm was used, which allowed us to gather (1) behavioral measures during go trials; and (2) ERP unaffected by motor artifacts during no-go trials. Specifically, on go trials, participants were instructed to press a button on the response pad with their right or left index finger when the orientation of the bar probe (horizontal or vertical) matched the orientation of the thicker line of the fixation cross at the time of probe onset (i.e., one line of the fixation cross was thickened). Participants were to withhold this response on no-go trials (i.e., when neither line of the fixation cross was thickened). In each block 24 go-trials and 56 no-go trials were presented (total 30% go and 70% no-go trials).
Participants were instructed to focus on the fixation cross while concurrently monitoring the orientation of the probe. Response time was recorded from probe onset. Trials with response times that were <100 ms and >1500 ms were excluded from the analyses. Accuracy was measured as the number of correct responses to go stimuli (“hits”), the number of incorrect responses to no-go stimuli (“false-alarms”) and the number of incorrect responses to go stimuli (“misses”).
EEG recording and data reduction
EEG was recorded continuously using a 128-channel Electrical Geodesics system (EGI Inc, Eugene, OR) at 500 Hz with 0.1-200 Hz analog filtering referenced to the vertex. Impedance of all channels was kept below 50 kΩ. Data were processed using Brain Vision Analyzer (Brain Products GmbH, Germany). Data were resampled at 250 Hz, segmented and re-referenced off-line to an average reference. Continuous EEG was manually inspected for gross movement artifact and artifact from each segment was later removed automatically with a ±75 µV criterion on a channel-by-channel basis. Eye-movement artifacts, particularly blinks, were corrected by Independent Component Analysis. EEG epochs were extracted beginning 100 ms before and ending 350 ms after stimulus presentation. Averages were low-passed filtered at 30 Hz and the amplitude of the ERP was derived from each individual’s average waveform. A pre-response baseline between −100 to 0 ms was used for all ERP components.
Only ERP components for no-go trials were analyzed to avoid contamination from motor artifacts. Three ERP components time-locked to face pair onset were identified: the C1, P1 and N170. The C1 was measured as the most negative peak in the time window of 50–80 ms following face onset at midline occipital parietal sites (channels 68, 73 on the EGI net). The C1 was maximal at channel 73. The P1 was measured as the most positive peak in the time window of 80–150 ms following face onset at left and right occipital/posterior sites (channels: left 59, 60, 66, 71; right 92, 86, 85, 84). The P1 was maximal at channel 92 (right). The N170 was measured as the most negative peak in the time window of 130–210 ms following face onset at left and right occipital temporal sites (channels left: 51, 58, 59, 64, 65; right 98, 97, 92, 96, 91). The N170 was maximal at channel 92.
Three ERP components time-locked to the probe were identified: the C1, P1 and N1. The C1 was maximal at channel 73. The P1 following probe onset was again maximal at channel 92. The N1 was measured as the most negative peak in the time window of 150–250 ms following probe onset at left and right posterior sites (channels left: 60, 66 67, 71; right: 86, 85, 78, 84). The N1 was maximal at 85 (right). All maximal electrode sites were contralateral to the visual field of stimulus presentation.
LORETA whole brain analyses
In cases of significant scalp ERP findings, Low Resolution Electromagnetic Tomographhy (LORETA; Pascual-Marqui, Michel, & Lehmann, 1994) was used to estimate intracerebral current density underlying the scalps effects. The LORETA algorithm is a form of Laplacian-weighted minimal norm solution that solves the inverse problem by assuming that: (1) neighboring neurons are synchronously activated and display only gradually changing orientations; and (2) the scalp-recorded signal originates mostly from cortical gray matter. The LORETA algorithm does not assume an a priori number of underlying sources to solve the inverse problem. Independent validation for the algorithm has been derived from studies combining LORETA with fMRI (Mulert, Jager, Schmitt, Bussfeld, Pogarell, Moller, et al., 2004; Vitacco, Brandies, Pascual-Marqui, & Martin, 2002), PET (Pizzagalli, Oakes, Fox, Chung, Larson, Abercrombie, et al., 2004; but see Gamma, Lehmann, Frei, Iwata, Pascual-Marqui, & Vollenweider, 2004), and intracranial recordings (Zumsteg, Friedman, Wennberg, & Wieser, 2005). LORETA localizations have been reported to be, on average 16 mm (Mulert et al., 2004) and 14.5 mm (Vitacco et al., 2002) from fMRI activation loci, a discrepancy within the range of the LORETA’s estimated spatial resolution (~1–2 cm).
For the present study, a three-shell spherical head model registered to the Talairach brain atlas (available as digitized MRI from the Brain Imaging Centre, Montreal Neurological Institute - MNI) and EEG electrode coordinates derived from cross-registrations between spherical and realistic head geometry (Towle et al., 1993) were used. The solution space (2,394 voxels; voxel resolution: 7 mm3) was constrained to cortical gray matter and hippocampi, which were defined according to a digitized probability atlas provided by the MNI (i.e., coordinates reported in main text are in MNI space). After converting MNI coordinates into Talairach space (Brett et al., 2002), the Structure-Probability Maps atlas (Lancaster et al., 1997) was used to identify gyri and Brodmann area(s).
Based on findings from the scalp ERP analyses, LORETA source localization for the P1 validity effect was computed within an 84–124 ms post-stimulus time window which was centered +/− 20 ms around the global field power peak (GFP) which indexes time points associated with maximal neuronal activity, and thus offer optimal signal-to-noise ratio (Lehmann & Skrandies, 1984). The GFP peak for the P1 was 104 ms, coinciding with the mean P1 latency (100.69 ms, SD = 9.35 ms). At each voxel, current density was computed as the linear, weighted sum of the scalp electric potentials (units are scaled to amperes per square meter, A/m2). For each subject, LORETA values were normalized to a total power of 1 and then log transformed before statistical analyses.
Statistical analyses
For response time, a Visual Field (LVF, RVF) by Validity (Valid, Invalid) by Emotion (Angry, Happy) analysis of variance (ANOVA) was performed [Participants made very few errors in this task, precluding statistical analyses on hit rates.] For ERPs time-locked to the face presentation, a 2 × 2 × 2 ANOVA using Hemisphere (left, right), Visual Field, and Emotion ANOVA as repeated measures was performed on ERP amplitudes at the right sensor showing the largest amplitude and the homologous left sensor. For ERPs time-locked to the bar probe, a Hemisphere by Visual Field by Validity by Emotion ANOVA was performed at the maximal right and corresponding left site. For the sake of brevity, only effects involving the factor Emotion were fully evaluated. All results reported are Greenhouse-Geisser corrected. Paired t-tests (two-tailed) were performed to decompose significant interactions. Pearson correlation analyses were also performed to examine the relation between P1 validity effects and the C1 component (see Pourtois et al., 2004). Throughout the analyses, two-tailed tests were used.
Results
Behavioral analyses
Participants made an average of 1.63 misses on go trials (SD 1.58) and 4.67 (SD 2.91) false alarms on no-go trials. An ANOVA for response time revealed a significant Emotion by Validity interaction, F(1, 14) = 22.69, p < .001, such that participants were faster to respond to the probe when it appeared in the same location as the angry face (i.e., valid cue; M = 507.64, SD = 49.93) compared to when it appeared in the opposite location (i.e., invalid cue; M = 521.88 , SD = 57.24), t(14) = 4.09, p = .001. An opposite pattern was found for happy faces (valid cue: M = 517.29, SD = 50.21; invalid cue: M = 504.51, SD = 54.12), t(14) = 3.39, p = .004. Finally, on valid trials, probe RTs following angry faces were faster than happy faces, t(14) = 2.50, p = .027, while on invalid trials, probe RTs following happy faces were faster than angry faces, t(14) = 4.36, p = .001 (see Figure 2).
Figure 2.
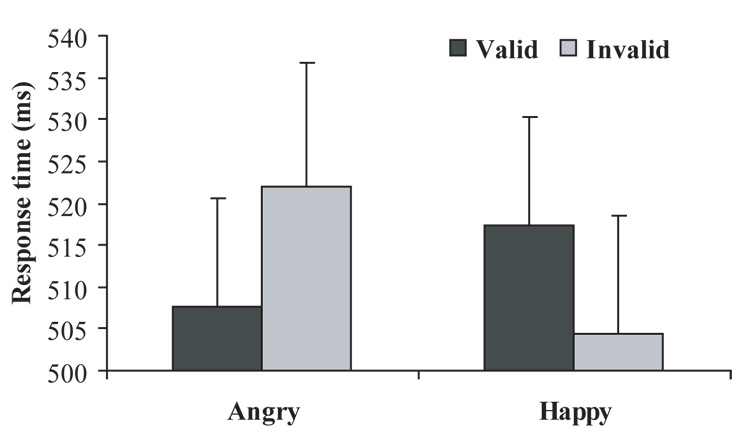
Response times for the dot-probe task illustrating a Validity by Emotion interaction (p < .001). Bars represent SE.
ERP analyses
ERPs time-locked to the faces
Table 1A lists the mean (and SE) amplitudes, while Figure 3A displays the topographic maps for the ERP components elicited by angry and happy face pairs (for a given picture, the facial expression was presented with a corresponding neutral expression).
Table 1.
| Table 1A Mean (SE) amplitude (µV) of the C1 (latency: 69.2 ± 11.0), P1 (106.7 ± 8.3), and N170 (170.2 ± 15.5) time-locked to face presentation at the left and right maximal sites | ||||
|---|---|---|---|---|
| Visual Field | Emotion | Component | ||
| C1 | P1 | N170 | ||
| Left | Angry | −1.01 (.25) | 1.83 (.30) | −2.66 (.33) |
| Happy | −1.17 (.25) | 1.80 (.29) | −2.66 (.31) | |
| Right | Angry | −.99 (.29) | 1.81 (.30) | −2.85 (.35) |
| Happy | −.84 (.32) | 1.83 (.34) | −2.75 (.34) | |
| Table 1B Mean (SE) amplitude (µV) of the C1 (latency: 61.4 ± 8.0), P1 (100.9 ± 9.3), and N1 (216.6 ± 16.6) time-locked to probe presentation at the left and right maximal sites | ||||
|---|---|---|---|---|
| Emotion | Validity | Component | ||
| C1 | P1 | N1 | ||
| Angry | Valid | −.44 (.10) | 1.85 (.20) | −4.06 (.48) |
| Invalid | −.43 (.15) | 1.61 (.20) | −4.03 (.49) | |
| Happy | Valid | −.53 (.14) | 1.65 (.18) | −4.11 (.53) |
| Invalid | −.54 (.16) | 1.85 (.26) | −4.06 (.51) | |
Figure 3.
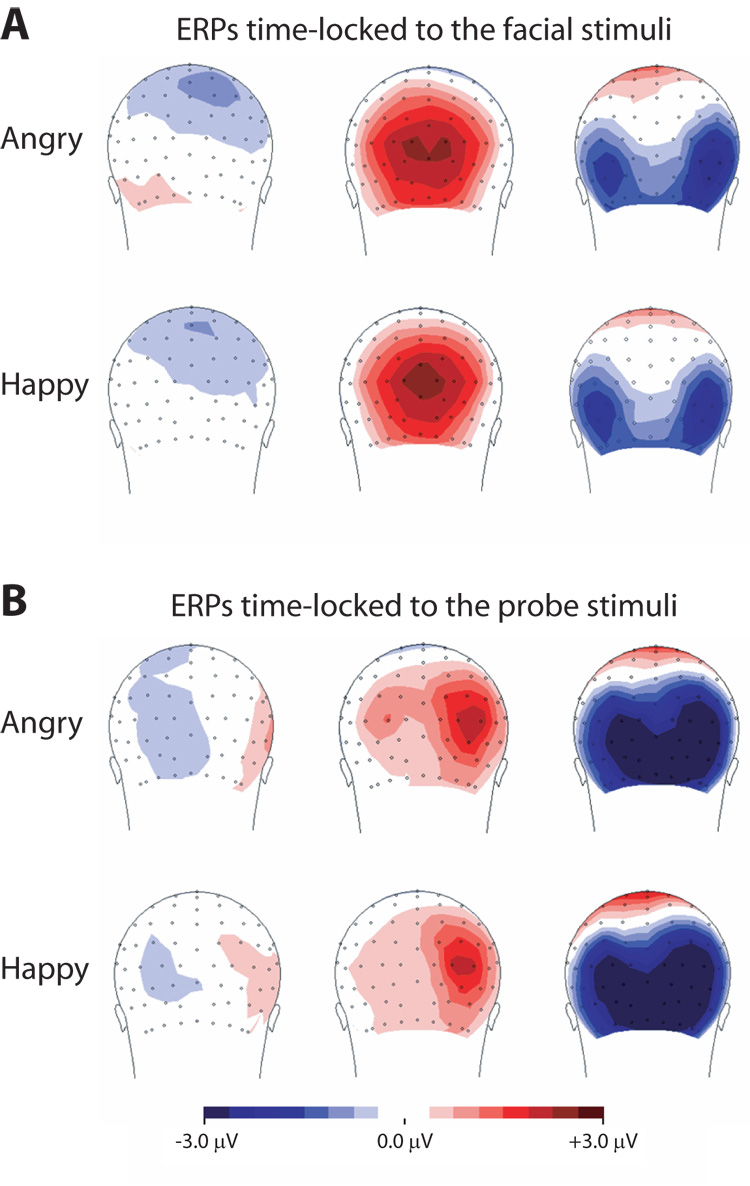
(A) ERPs time-locked to the faces: Topographic maps of the C1, P1 and N170 peak elicited by the angry (top) and happy (bottom) face pairs presented in the LVF; (B) ERPs time-locked to the probes: Topographic maps of the C1, P1 and N1 peak elicited by the probes following validly cued angry (top) and happy (bottom) face pairs in the LVF.
C1 amplitude
An ANOVA for the C1 evoked by the face pairs revealed no significant main effects or interactions (all Fs < 1.63, all ps > .22).
P1 amplitude
No significant main effects or interactions emerged when considering the P1 elicited by face pairs (all Fs < 3.27, all ps > .09).
N170 amplitude
An ANOVA for the N170 elicited by face pairs revealed a main effect for Hemisphere, F(1, 14) = 4.90, p = .044, such that the N170 was larger in the right hemisphere (M = 3.15, SD = 1.14) compared with the left hemisphere (M = −2.31, SD = 1.71) regardless of the emotion or the field of visual presentation. There was no difference in the amplitude of the N170 for angry versus happy faces. No other significant main effects or interactions were found.
Taken together, ERPs time-locked to the facial stimuli did not reveal any affect-modulated findings.
ERPs time-locked to the probes
Table 1B displays the mean (and SE) amplitudes of ERPs elicited by the probe whereas Figure 3B displays the topographic maps for the ERP components elicited by validly cued probes following angry and happy face pairs.
C1 amplitude
For the C1, no significant main effects or interactions were found (all Fs < .33, all ps > .58).
P1 amplitude
The P1 elicited by the probe was localized primarily over the extrastriate occipal regions (BA 19). An ANOVA revealed a significant main effect of Hemisphere, F(1, 14) = 8.26, p = .012, such that, as expected, the P1 was largest in the right than left hemisphere. Of particular interest, there was also a significant Validity by Emotion interaction, F(1, 14) = 4.77, p = .046 (Figure 4). Follow-up tests indicated this effect was due to the P1 being significantly larger for valid (M = 1.85, SD = .78) than invalid (M = 1.61, SD = .78) probes following angry face pairs, t(14) = 2.23, p = .042 (Figure 5A). LORETA paired-sample t-tests of this effect revealed significantly greater activity in the anterior cingulate cortex (BA 24, 32, 9) in response to invalidly compared with validly cued angry probes (Table 1 and Figure 5B). In contrast, valid angry trials were associated with greater activity in the orbital frontal cortex (OFC)/superior frontal gyrus (BA 10, 11), but this effect was restricted to only four voxels.
Figure 4.
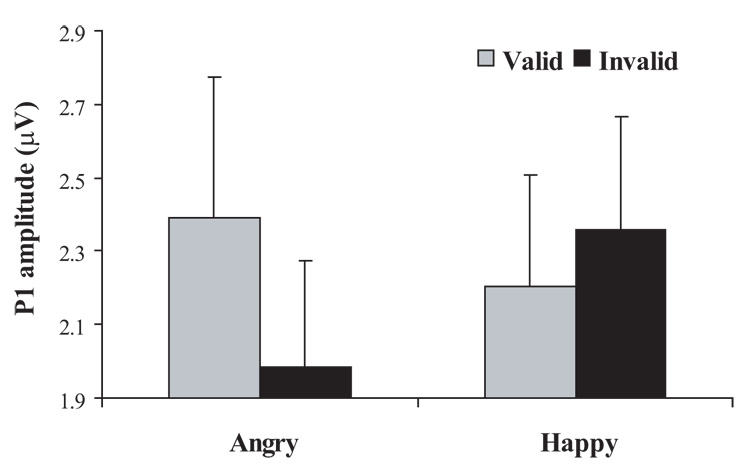
ERPs time-locked to the probes: P1 amplitudes to the probe illustrating a Validity by Emotion interaction (p = .046). Bars represent SE.
Figure 5.
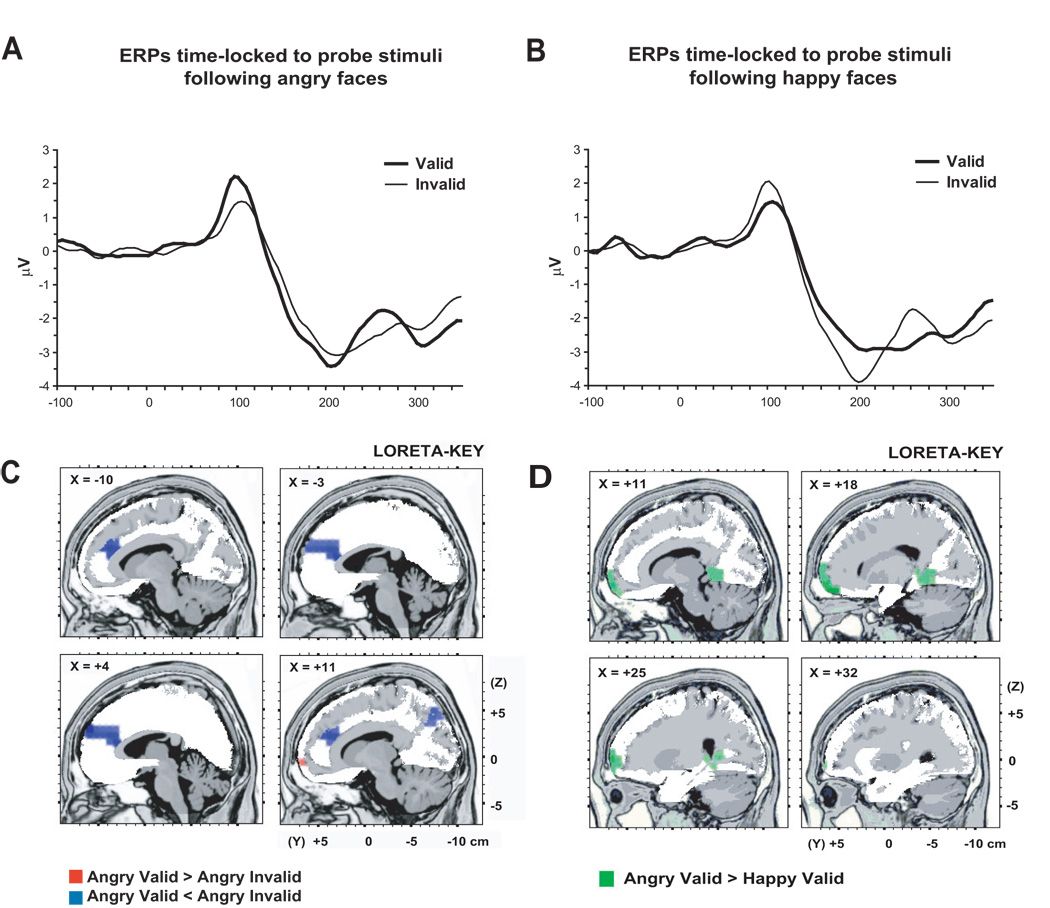
(A) ERPs time-locked to the probes: Grand average ERP waverforms for the P1 to valid (light line) and invalid (heavy line) probes following angry faces (p = .042) presented in the LVF at channel 92 (right posterior electrode). (B) Results of voxel-by-voxel paired t-tests contrasting current density for the P1 elicited by valid versus invalidly cued probes following an angry face presentation. Red: angry valid > angry invalid. Blue: angry valid < angry invalid. (C) ERPs time-locked to the probes: Grand average ERP waverforms for the P1 to valid (light line) and invalid (heavy line) probes following happy faces (p = .038) presented in the RVF at channel 92 (right posterior electrode). (D) Results of voxel-by-voxel paired t-tests contrasting current density for the P1 elicited by valid cued probes following angry face versus happy face presentation. Green: angry > happy. In panel (B) and (D), statistical maps are thresholded at p < 0.01 and displayed on the MNI template.
For happy faces presented in the RVF (i.e., neutral faces in the LVF), the P1 was larger for invalidly cued probes than validly cued probes, t(13) = 2.30 p = .038 (Figure 5C). LORETA paired t-tests of this difference failed, however, to reveal any significant findings. Finally, there was also a trend showing that the P1 for validly cued angry probes was larger than the P1 for validly cued happy probes, t(13) = 1.8, p = .09. This effect was much stronger, however, using a LORETA paired-sample t-test, which indicated that validly cued angry trials was associated with greater activity than validly cued happy trials in the OFC/superior frontal gyrus (BA 10, 11) as well as the lingual and parahippocampal gyri (BA 19, 30; Table 1 and Figure 5D). Finally, unlike the findings reported by Pourtois et al. (2004), validly cued probes following angry (or happy) face pairs were not associated with significantly enhanced activity in extrastriate areas (see also Figure 3B).
N1 amplitude
For the N1 elicited by the probe, there was a significant Hemisphere by Visual Field interaction, F(1, 14) = 5.93, p = .029. There was also a significant Hemisphere by Emotion interaction, F(1, 14) = 8.96, p = .01, with probes following happy faces eliciting slightly larger N1s in the left hemisphere than angry faces, t(14) = 1.85, p = .085. No other significant main effects or interactions were found.
Taken together, findings emerging from ERPs time-locked to the probe revealed that earliest, and only, index of spatial attention was manifested in the P1 peaking at 100 ms which was modulated by emotional faces.
Correlations between ERP components
Following Pourtois et al. (2004), “validity effect” of the P1 was calculated as the amplitude difference between valid and invalid trials. This effect was correlated with the C1 elicited by the face pairs to determine if early facial processing predicted enhanced processing of validly cued probes. There was no significant relation (r = −.12, p > .67).
Discussion
The purpose of the present study was to examine the brain mechanisms underlying involuntary orienting using a dot-probe task with angry face pairs in healthy adults. To our knowledge, this topic has not been investigated before, and findings emerging from this study thus provide novel information about processing underlying involuntary orienting towards cues of social threat and/or disapproval elicited by angry faces in healthy participants. Consistent with previous research using a rapid presentation time (100 ms) and short SOAs, response time was facilitated for validly cued probes following angry faces compared with happy faces (Bradley et al., 2000; Cooper & Langton, 2004; Mogg et al., 2004). An opposite pattern was found for happy faces such that response times were faster for invalidly cued happy faces or, alternatively, validly cued neutral faces. Both of these results are plausible, if we assume that attention was directed toward the relatively more threatening stimulus on the screen (Cooper & Langton, 2006; Mogg & Bradley, 1999).
Complementing these behavioral findings, the earliest electrophysiological index of spatial attention was manifested in the P1. Similar to Pourtois et al. (2004), the P1 was larger for validly cued probes following angry faces than invalid probes, confirming that threatening cues can modulate spatial attention in healthy adults. Greater brain activity observed in the anterior rostral cingulate area (BA 24, 32) to invalidly cued probes following angry faces is consistent with the role of this region in detecting conflicts in information processing (e.g., overriding prepotent responses, response competition, attentional control; Botvinick et al., 2004; Bush et al., 2000). In this case, interference might have occurred for probe detection when attention was directed to an angry face in the opposite location.
Consistent with the response time performance presented here and by others (Cooper and Langton, 2006), larger P1s were observed for invalidly cued probes following happy faces compared with validly cued probes or again, for validly cued neutral faces. It is unclear, however, whether attention was initially directed towards the most threatening or salient face (i.e., angry in angry/neutral pair and neutral in happy/neutral pair) or if participants actually shifted attention away from the happy face – and it is only at longer presentation durations or SOAs that a bias towards the happy faced would occur. Unfortunately, we do not have self-report ratings of saliency for each face stimulus and, unlike Cooper and Langton (2006), did not examine the effect of longer SOAs. Recent fMRI studies have reported, however, that activation in the amygdala is not specific to threat detection, but is activated for socially salient stimuli, even neutral faces (Fitzgerald et al., 2006; Klienhans et al., 2007; Sander, Grafmann & Zalla, 2003). Although amygdala activation cannot be measured by ERPs, presumed functional outputs of amygdala activation such as the ACC, medial and lateral prefrontal cortices and occipital cortex, are measurable at the scalp (Blair, Morris, Frith, Perrett, & Dolan, 1999; Morris, Friston, Buechel, Frith, Young, Calder, & Dolan, 1998; Vuilleumier & Pourtois, 2007). Future studies are needed to replicate this P1 effect with happy/neutral face stimuli and confirm the pattern of activation revealed by LORETA source localization. Moreover, it is unclear why invalidly cued happy probes did not activate the same ACC region as invalidly cued angry trials since the conflict experienced would be equivalent.
The P1 for validly cued angry probes was also slightly larger and was associated with greater activity in the OFC/superior frontal gyrus (BA 10, 11) than the P1 for validly cued happy probes. The pattern of activity in this region seen here, and for validly cued versus invalidly cued angry probes, is consistent with work demonstrating a role for the orbitofrontal cortex and adjacent prefrontal areas in social and emotional responses to faces (Rolls, Critchley, Browning, & Inoue, 2006; Wilson, O’Scalaidhe, & Goldman-Rackic, 1993). These areas are not only implicated in decoding the social information and social reinforcement conveyed in emotional expressions but also in using this information to guide social interactions (for review see Rolls, 2007).
The C1 elicited by face stimuli was not modulated by emotional valence, nor did it predict subsequent validity effects on the P1, which is inconsistent with Pourtois et al., (2004). It is, however, unclear how the use of angry versus fearful faces may have altered our results. The C1 to the probe was also not modulated by attention, consistent with previous research (e.g., Clark & Hillyard, 1996; Di Russo, Martinez, & Hillyard, 2003; Mangun, 1995; Martinez, Anllo-Vento, Sereno, Frank, Buxton, Dubowitz et al., 1999). Taken together, results suggest the C1 component is not a reliable index of early emotion-related neural activation arising from the primary visual cortex or selective attention towards emotionally significant stimuli. Of course, as Hillyard et al. (2004) pointed out, activation of the primary visual cortex might not be time-locked to the attended stimuli and does not appear in ERP waveforms, making initial responses to visual stimuli difficult to measure.
Consistent with Pourtois and others, the N170 to face stimuli was not modulated by emotional valence (Eimer, Holmes, & McGlone, 2003; Holmes, Winston, & Eimer, 2005; Pourtois et al., 2004) and the N1 to the probe was not modulated by cue validity (Pourtois et al., 2004), although the amplitude of the N1 followed the same direction as the P1. Others have reported, however, larger N170 amplitudes for angry faces (Kolassa & Milner, 2006), fearful faces (Blau et al., 2007), and personally salient faces (Pizzagalli et al., 2002) compared with other emotional expressions and larger N1 amplitudes have been repeatedly associated with greater attentional processing (Clark & Hillyard, 1996; Di Russo, et al., 2003; Mangun, 1995; Mangun, Hinrichs, Scholz, Mueller-Gaerner, Herzog, Krause et al., 2001; Martinez, Di Russo, Anllo-Vento, Sereno, Buxton & Hillyard, 2001; Martinez et al., 1999). However, given differences in source localization and dissociations observed for these components under various attentional manipulations (which were not measured here), the P1 and N1 might reflect distinct aspects of spatial attention (Hillyard, Luck, & Mangun, 1994; Luck, 1994; Mangun & Hillyard, 1990). It is currently proposed that the P1 reflects processing facilitation (via sustained gating of input at an attended location, bias, or suppression of information at the unattended location), whereas the N1 reflects the operation of a discrimination process within the focus of attention (see Natale, Marzi, Girelli, Pavone, & Pollmann, 2007; Vogel & Luck, 2000).
The present study is not without limitations. First, self-report measures of the threat-value/saliency as well as state anxiety were not collected. Others have noted that threat-value, in combination with one’s state anxiety level, influence attentional bias. Specifically, higher rated threat relevance and higher anxiety have been associated with greater attentional bias towards negative stimuli (Bradley, Mogg, & Millar, 2000; Georgiou, Bleakley, Hayward, Russo, Dutton, Eltiti, & Fox, 2005; but see also Frewen, Dozois, Joanisse, & Neufeld, in press).
Second, the present study did not examine other negatively valenced faces (e.g., sad, fearful), limiting comparisons to prior ERP studies using similar paradigms (e.g., Portious et al., 2002). A variety of emotional expressions (fear, disgust, angry, sad, neutral and happy) may or may not activate the amygdala (Fitzgerald et al., 2006). The strength of amygdala output signals to distinct neural circuits depends on the valence, meaning, and functional impact of the stimulus (Matthews, Mackintosh, & Fulcher, 1997; Springer, Rosas, McGetrick, & Bowers, 2007). Moreover, Williams et al. (2005) reported that during a visual search paradigm using a variety of emotions, angry and happy faces were detected faster than fearful and sad faces (with angry faces showing a superiority effect). The authors argued that fearful faces may convey a different kind of threat than angry faces: the former conveying threat from an individual, drawing focal attention; the latter conveying environmental threat, drawing attention elsewhere. Determining how various emotional expressions modulate attentional bias, and how they depend on anxiety level and/or perceived threat might be particularly important for elucidating the mechanisms underlying the development and maintenance of anxiety disorder, particularly social anxiety disorders (see Matthews & MacLeod, 1994, 2002).
Third, as with any dot-probe task, only a “snapshot” of attention was obtained – that is, where attention was directed when the probe appeared. Without a more direct measure of attention (e.g., visual scanpath, eye tracking), we cannot be certain where attention was allocated just before or after probe presentation and, whether attention was captured by the angry and neutral faces in the angry/neutral, happy/neutral face pairs or directed away from happy faces in the happy/neutral face pair. Finally, no baseline measure of attentional control was examined. It is possible that individual differences in attentional control mediated, in part by, the ACC moderate attentional biases on the dot-probe task (see Frewen et al., in press).
In summary, the present study demonstrates that threatening facial expressions (angry faces) modulate spatial attention in healthy adults. Validity effects were found for both response time performance and P1 amplitude. ERP source localization confirmed that the earliest electrophysiological index of spatial attention was localized to extrastriate/occipital regions around 100 ms possibly reflecting top-down influences from prefrontal regions and/or direct influences from face/threat-sensitive regions, such as the amygdala. These results validate recent findings that angry faces presented rapidly effectively capture involuntary attention (Cooper & Langton, 2006) and may provide a promising framework for probing attentional biases that have been implicated in the etiology and maintenance of emotional disorders, including social anxiety disorder.
Table 2.
Summary of significant results emerging from whole-brain LORETA analyses 84–124 ms after valid versus invalidly cued probes following angry face pairs.
| Region | MNI Coordinates (mm) | Brodmann's | Voxels | t-value | p-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | Area (s) | ||||
| Valid versus invalid angry | |||||||
| ACC/Medial frontal gyrus | 4 | 59 | 29 | 24,32,9 | 35 | −4.76 | 0.0004 |
| Superior frontal gyrus | 18 | 66 | 6 | 10,11 | 4 | 3.52 | 0.0038 |
| Valid angry versus valid happy | |||||||
| Superior frontal gyrus | 18 | 59 | −20 | 10,11 | 18 | 4.63 | 0.0005 |
| Lingual/Parahippocampal gyri | 11 | −53 | 1 | 19,30 | 15 | 3.54 | 0.0036 |
Note. The anatomical regions, MNI coordinates, and BAs of extreme t-values are listed. Positive t-values are indicative of stronger current density for e.g. validly cued angry probes than invalidly cued angry probes, and vice versa for negative t-values. The numbers of voxels exceeding the statistical threshold are also reported (p < 0.01). Coordinates in mm (MNI space), origin at anterior commissure; (X) = left (−) to right (+); (Y) = posterior (−) to anterior (+); (Z) = inferior (−) to superior (+).
Acknowledgments
This work was partially supported by a NIMH grant R01MH68376 awarded to Diego A. Pizzagalli. Dr. Hofmann is supported by NIMH grant R01MH078308. The authors are grateful to Allison Jahn and James O’Shea for their skilled assistance at early stages of this project, and to Dr. Pourtois for providing the stimuli used in the present study.
Erik M. Mueller is currently at the Department of Psychology, University of Marburg, Marburg, Germany. Kyle G. Ratner is currently at the Department of Psychology, New York University, USA. Etienne B. Roesch is currently at the Swiss Centre for Affective Sciences (CISA), University of Geneva, Geneva, Switzerland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures Dr. Pizzagalli has received research support from GlaxoSmithKline and Merck & Co., Inc. for projects unrelated to the present study. Dr. Hofmann is a paid consultant by Organon for issues and projects unrelated to this study. Drs. Santesso and Meuret as well as Mr. Mueller, Ratner, and Roesch report no competing interests.
References
- Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. Journal of Cognitive Neuroscience. 2002;14:1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- Argyle M. The Psychology of Interpersonal Behaviour. 4th ed. Middlesex, England: Penguin Books; 1983. [Google Scholar]
- Averill JR. Studies on anger and aggression. Implications for theories of emotion. American Psychologist. 1983;38:1145–1160. doi: 10.1037//0003-066x.38.11.1145. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Glickman S. Attentional bias in anxiety: A behavioral and ERP study. Brain and Cognition. 2005;59:11–22. doi: 10.1016/j.bandc.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blau VC, Maurer U, Tottenham N, McCandliss BD. The face-specific N170 component is modulated by emotional facial expression. Behavioral and Brain Functions. 2007;3:7. doi: 10.1186/1744-9081-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Millar NH. Covert and overt orienting of attention to emotional faces in anxiety. Cognition and Emotion. 2000;14:789–808. [Google Scholar]
- Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nature Reviews. Neuroscience. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of handedness. Brain and Cognition. 1987;6:175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Clark VP, Hillyard SA. Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. Journal of Cognitive Neuroscience. 1996;8:387–402. doi: 10.1162/jocn.1996.8.5.387. [DOI] [PubMed] [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social phobia: diagnosis, assessment, and treatment. New York: Guilford Press; 1995. pp. 69–93. [Google Scholar]
- Cooper RM, Langton SRH. Attentional bias to angry faces using the dot-probe task? It depends when you look for it. Behaviour Research and Therapy. 2006;44:1321–1329. doi: 10.1016/j.brat.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martínez A, Hillyard SA. Source analysis of event-related cortical activity during visuo-spatial attention. Cerebral Cortex. 2003;13:486–499. doi: 10.1093/cercor/13.5.486. [DOI] [PubMed] [Google Scholar]
- Eastwood JD, Smilek D, Merikle PM. Differential attentional guidance b by unattended faces expressing positive and negative emotion. Perception and Psychophysics. 2001;6:1004–1013. doi: 10.3758/bf03194519. [DOI] [PubMed] [Google Scholar]
- Eimerq M. “Sensory gating” as a mechanism for visuospatial orienting: electrophysiological evidence from trial-by-trial cuing experiments. Perception and Psychophysiology. 1994;55:667–675. doi: 10.3758/bf03211681. [DOI] [PubMed] [Google Scholar]
- Eimer M, Holmes A. Event-related brain potential correlates of emotional face processing. Neuropsychologia. 2007;45:15–31. doi: 10.1016/j.neuropsychologia.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M, Holmes A, McGlone F. The role of spatial attention in the processing of facial expression: An ERP study of rapid brain responses to six basic emotions. Cognitive, Affective, and Behavioral Neuroscience. 2003;3:97–110. doi: 10.3758/cabn.3.2.97. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Fox E, Lester V, Russo R, Bowles RJ, Pichler A, Dutton K. Facial expressions of emotions: Are angry faces detected more efficiently? Cognition and Emotion. 2000;14:61–92. doi: 10.1080/026999300378996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Joanisse MF, Neufeld RWJ. Selective attention to threat versus reward: Meta-analysis and neural-network modeling of the dot-probe task. Clinical Psychology Review. doi: 10.1016/j.cpr.2007.05.006. in press. [DOI] [PubMed] [Google Scholar]
- Fu S, Fan S, Chen L, Zhuo Y. The attentional effects of peripheral cueing as revealed by two event-related potential studies. Clinical Neurophysiology. 2001;112:172–185. doi: 10.1016/s1388-2457(00)00500-9. [DOI] [PubMed] [Google Scholar]
- Fu S, Caggiano DM, Greenwood PM, Parasuraman R. Event-related potentials reveal dissociable mechanisms for orienting and focusing visuospatial attention. Cognitive Brain Research. 2005;23:341–353. doi: 10.1016/j.cogbrainres.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamma A, Lehmann D, Frei E, Iwata K, Pascual-Marqui RD, Vollenweider FX. Comparison of simultaneously recorded [H2(15)O]-PET and LORETA during cognitive and pharmacological activation. Human Brain Mapping. 2004;22:83–96. doi: 10.1002/hbm.20015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou GA, Bleakley C, Hayward J, Russo R, Dutton K, Eltiti S, Fox E. Focusing on fear: Attentional disengagement from emotional faces. Visual Cognition. 2005;12:145–158. doi: 10.1080/13506280444000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Raij T, Marinkovic K, Jousmaeki V, Hari R. Cognitive response profile of the human fusiform face area as determined by MEG. Cerebral Cortex. 2000;10:69–81. doi: 10.1093/cercor/10.1.69. [DOI] [PubMed] [Google Scholar]
- Heinrichs N, Hofmann SG. Information processing in social phobia: a critical review. Clinical Psychology Review. 2001;21:751–770. doi: 10.1016/s0272-7358(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Luck SJ, Mangun GR. The cuing of spatial attention to visual field locations: Analysis with ERP recordings. In: Heinze HJ, Munte TF, Mangun GR, editors. Cognitive electrophysiology. Boston, MA: Birkhauser; 1994. pp. 1–25. [Google Scholar]
- Holmes A, Vuilleumier P, Eimer M. The processing of emotional facial expression is gated by spatial attention: evidence from event-related brain potentials. Cognitive Brain Research. 2003;16:174–184. doi: 10.1016/s0926-6410(02)00268-9. [DOI] [PubMed] [Google Scholar]
- Holmes A, Winston J, Eimer M. The role of spatial frequencyinformation for ERP components sensitive to faces and emotional facial expression. Cognitive Brain Research. 2005;25:508–520. doi: 10.1016/j.cogbrainres.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Horley K, Williams LM, Gonsalvez C, Gordon E. Face to face: visual scanpath evidence for abnormal processing of facial expressions in social phobia. Psychiatry Research. 2004;127:45–53. doi: 10.1016/j.psychres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Horstmann G, Bauland A. Search asymmetries with real faces: Testing the anger-superiority effect. Emotion. 2006;6:193–207. doi: 10.1037/1528-3542.6.2.193. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Johson LC, Mahurin R, Richards T, Stegbauer KC, Greenson J, Dawson G, Aylward E. Increased amygdala activation to neutral faces is associated with better face memory performance. Neuroreport. 2007;18:987–991. doi: 10.1097/WNR.0b013e328165d189. [DOI] [PubMed] [Google Scholar]
- Kolassa IT, Miltner WH. Psychophysiological correlates of face Processing in social phobia. Brain Research. 2006;1118:130–141. doi: 10.1016/j.brainres.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Massiotta JC. Automated labeling of the human brain—a preliminary report on the development and evaluation of a forward-transformed method. Human Brain Mapping. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D, Skandries W. Spatial analysis of evoked potentials in man--a review. Progress in Neurobiology. 1984;23:227–250. doi: 10.1016/0301-0082(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Liu J, Harris A, Kanwisher N. Stages of processing in face perception: an MEG study. Nature Neuroscience. 2002;5:910–916. doi: 10.1038/nn909. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and of macaque visual cortex. Journal of Neurophysiology. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Mangun GR. Neural mechanisms of visual selective attention. Psychophysiology. 1995;32:4–18. doi: 10.1111/j.1469-8986.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Allocation of visual attention to spatial locations: trade off functions for event-related brain potentials and detection performance. Perception and Psychophysiology. 1990;47:532–550. doi: 10.3758/bf03203106. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hinrichs H, Scholz M, Mueller-Gaertner HW, Herzog H, Krause BJ, Tellman L, Kemna L, Heinze HJ. Integrating electrophysiology and neuroimaging of spatial selective attention to simple isolated visual stimuli. Vision Research. 2001;41:1423–1435. doi: 10.1016/s0042-6989(01)00046-3. [DOI] [PubMed] [Google Scholar]
- Martínez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nature Neuroscience. 1999;2:364–369. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- Martínez A, Di Russo F, Anllo-Vento L, Sereno MI, Buxton R, Hillyard SA. Putting spatial attention on map: timing and localization of stimulus selection processes in striate and extrastriate visual areas. Vision Research. 2001;41:1437–1457. doi: 10.1016/s0042-6989(00)00267-4. [DOI] [PubMed] [Google Scholar]
- Matthews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annual Review of Psychology. 1994;45:25–50. doi: 10.1146/annurev.ps.45.020194.000325. [DOI] [PubMed] [Google Scholar]
- Matthews A, MacLeod C. Induced processing biases have causal effects on anxiety. Cognition and Emotion. 2002;16:331–354. [Google Scholar]
- Mathews A, Mackintosh B, Fulcher EP. Cognitive biases in anxiety and attention to threat. Trends in Cognitive Sciences. 1997;1:340–345. doi: 10.1016/S1364-6613(97)01092-9. [DOI] [PubMed] [Google Scholar]
- Mertens I, Siegmund H, Grusser OJ. Gaze motor asymmetries in the perception of faces during a memory task. Neuropsychologia. 1993;31:989–998. doi: 10.1016/0028-3932(93)90154-r. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behavior Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective attention and anxiety: A cognitive-motivational perspective. In: Power M, Dalgleish T, editors. Handbook of cognition and emotion. Chicester, UK: Wiley; 1999. pp. 145–170. [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113:160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buechel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Mulert C, Jager L, Schmitt R, Bussfeld P, Pogarell O, Moller HJ, Juckel G, Hegerl U. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage. 2004;22:83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Natale E, Marzi CA, Girelli M, Pavone EF, Pollmann S. ERP and fMRI correlates of endogenous and exogenous focusing of visual-spatial attention. European Journal of Neuroscience. 2007;23:2511–2521. doi: 10.1111/j.1460-9568.2006.04756.x. [DOI] [PubMed] [Google Scholar]
- Öhman A. Face the beast and fear the face: Animal and social fears as prototypes for evolutionary analyses of emotion. Psychophysiology. 1986;23:123–145. doi: 10.1111/j.1469-8986.1986.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. International Journal of Psychophysiology. 1994;7:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Regard M, Lehmann D. Rapid emotional face processing in the human right and left brain hemispheres: an ERP study. Neuroreport. 1999;10:1–8. doi: 10.1097/00001756-199909090-00001. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, Abercrombie HC, Shaefer SM, Benca RM, Davidson RJ. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Molecular Psychiatry. 2004;325:393–405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Lehmann D, Hendrick AM, Regard M, Pascual-Marqui RD, Davidson RJ. Affective judgments of faces modulate early activity (~160 ms) within the fusiform gyri. NeuroImage. 2002;16:663–677. doi: 10.1006/nimg.2002.1126. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vuilleumier P. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral Cortex. 2004;14:619–633. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P. Neural systems for orienting attention to the location of threat signals: An event-related fMRI study. NeuroImage. 2006;31:920–933. doi: 10.1016/j.neuroimage.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Rohner J. The time-course of visual threat processing: High trait anxious individuals eventually avert their gaze from angry faces. Cognition and Emotion. 2002;16:837–844. [Google Scholar]
- Rolls ET. The representation of information about faces in the temporal and frontal lobes. Neuropsychologia. 2007;45:124–143. doi: 10.1016/j.neuropsychologia.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Browning AS, Inoue K. Face-selective and auditory neurons in the primate orbitofrontal cortex. Experimental Brain Research. 2006;170:74–87. doi: 10.1007/s00221-005-0191-y. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: An evolved system for relevance detection. Reviews in the Neurosciences. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Springer US, Rosas A, McGetrick J, Bowers D. Differences in startle reactivity during the perception of angry and fearful faces. Emotion. 2007;7:516–525. doi: 10.1037/1528-3542.7.3.516. [DOI] [PubMed] [Google Scholar]
- Towle VL, Bolanos J, Suarez D, Tan K, Grzeszczuk R, Levin DN, Cakmur R, Frank SA, Spire JP. The spatial location of EEG electrodes: locating the best-fitting sphere relative to cortical anatomy. Electroencephalography and Clinical Neurophysiology. 1993;86:1–6. doi: 10.1016/0013-4694(93)90061-y. [DOI] [PubMed] [Google Scholar]
- Vitacco D, Brandies D, Pascual-Marqui RD, Martin E. Correspondence Of event-related potential tomography and functional magnetic resonance imaging during language processing. Human Brain Mapping. 2002;17:4–12. doi: 10.1002/hbm.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37:190–203. [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Walker-Smith GJ, Gale AG, Findlay JM. Eye movement strategies involved in face perception. Perception. 1977;6:313–326. doi: 10.1068/p060313. [DOI] [PubMed] [Google Scholar]
- Williams MA, Moss SA, Bradshaw JL, Mattingley JB. Look at me, I’m smiling: Visual search for threatening and non-threatening facial expressions. Visual Cognition. 2005;12:29–50. [Google Scholar]
- Wilson FAW, O’Scalaidhe SPO, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Zumsteg D, Friedman A, Wennberg RA, Wieser HG. Source localization of mesial temporal interictal epileptiform discharges: correlation with intracranial foramen ovale electrode recordings. Clinical Neurophysiology. 2005;116:2810–2818. doi: 10.1016/j.clinph.2005.08.009. [DOI] [PubMed] [Google Scholar]