Abstract
Choline is an essential nutrient needed for the structural integrity and signaling functions of cell membranes; for normal cholinergic neurotransmission; for normal muscle function; for lipid transport from liver; and it is the major source of methyl groups in the diet. Choline is critical during fetal development, when it influences stem cell proliferation and apoptosis, thereby altering brain and spinal cord structure and function and influencing risk for neural tube defects and lifelong memory function. Choline is derived not only from the diet, but from de novo synthesis as well. Though many foods contain choline, there is at least a twofold variation in dietary intake in humans. When deprived of dietary choline, most men and postmenopausal women developed signs of organ dysfunction (fatty liver or muscle damage), while less than half of premenopausal women developed such signs. Aside from gender differences, there is significant variation in the dietary requirement for choline that can be explained by very common genetic polymorphisms.
Keywords: phosphatidylcholine, homocysteine, betaine, hippocampus, fatty liver
INTRODUCTION
The 1998 Institute of Medicine recommendations on dietary reference intakes included estimates of the Adequate Intake of choline required by humans (57). Choline is derived not only from the diet, but as well from de novo synthesis of phosphatidylcholine catalyzed by phosphatidylethanolamine N-methyltransferase (PEMT) in the liver (165). When deprived of dietary choline, most adult men and postmenopausal women developed signs of organ dysfunction (fatty liver or muscle damage) (33, 159). Only 44% of premenopausal women developed such signs. The difference in requirement occurs because estrogen induces the PEMT gene and allows premenopausal women to make more of their needed choline endogenously. In addition, there is significant variation in the dietary requirement for choline that can be explained by common genetic polymorphisms. Choline is critical during fetal development, when it influences stem cell proliferation and apoptosis, thereby altering brain structure and function (5, 31, 75, 85, 86). Similarly it influences neural tube development (40, 112). In later life, choline deficiency causes fatty liver as well as liver and muscle damage (33, 159), and reduces the capacity to handle a methionine load, resulting in elevated homocysteine (34), a risk factor for cardiovascular disease (46). Though many foods contain choline (160, 162), there is at least a twofold variation in dietary intake in humans (39, 112).
NUTRITIONAL IMPORTANCE OF CHOLINE
Choline is a dietary component essential for normal function of all cells (158). It, or its metabolites, assures the structural integrity and signaling functions of cell membranes; it is the major source of methyl groups in the diet (one of choline's metabolites, betaine, participates in the methylation of homocysteine to form methionine); and it directly affects cholinergic neurotransmission and lipid transport from liver (158). In most mammals, prolonged (weeks to months) ingestion of a diet deficient in choline (and adequate though limited in methionine and folate content) has consequences that include hepatic, renal, pancreatic, memory, and growth disorders (158). Several comprehensive reviews of the metabolism and functions of choline have been published (68, 158).
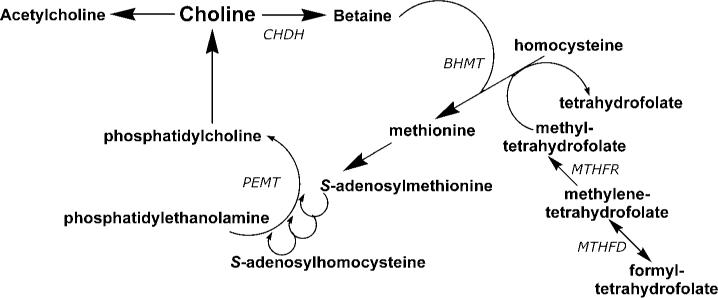
Only a small fraction of dietary choline is acetylated (Figure 1), catalyzed by the activity of choline acetyltransferase (reviewed in 12). This enzyme is highly concentrated in the terminals of cholinergic neurons, but is also present in such non-nervous tissues as the placenta. The availability of choline and acetyl-CoA influence choline acetyltransferase activity. In brain, it is unlikely that choline acetyltransferase is saturated with either of its substrates, so that choline (and possibly acetyl-CoA) availability determines the rate of acetylcholine synthesis (12). Increased brain acetylcholine synthesis is associated with an augmented release into the synapse of this neurotransmitter (26, 135, 146). Choline taken up by brain may first enter a storage pool (perhaps the phosphatidylcholine in membranes) before being converted to acetylcholine (10). The choline-phospholipids in cholinergic neurons are a large precursor pool of choline available for use in acetylcholine synthesis (11). This may be especially important in neurons with increased demands for choline to sustain acetylcholine release (e.g., when particular cholinergic neurons fire frequently, or when the supply of choline from the extracellular fluid is inadequate).
Figure 1.
Choline metabolism and links to methionine and folate metabolism. The pathways described are all present in liver, with other tissues having one or more of these pathways. BHMT, betaine homocysteine methyltransferase; CHDH, choline dehydrogenase; MTHFD, methylenetetrahydrofolate dehydrogenase; MTHFR, methylenetetrahydrofolate reductase; PEMT, phosphatidylethanolamine-N-methyltransferase.
The methyl groups of choline can be made available from one-carbon metabolism, upon conversion to betaine (reviewed in 95) (Figure 1). Formation of betaine involves oxidation to betaine aldehyde in the inner mitochondrial membrane (72) and then the oxidation of betaine aldehyde to form betaine; both oxidation steps are catalyzed by choline dehydrogenase. Liver and kidney are the major sites for choline oxidation. Betaine cannot be reduced back to choline. Thus, the oxidation pathway acts to diminish the availability of choline to tissues while, at the same time, scavenging some methyl groups. The interrelationship of this pathway with folate and methionine metabolism is discussed below.
A major use for choline is as a precursor for the synthesis of membrane phospholipids. Phosphatidylcholine (sometimes called lecithin) is the predominant phospholipid (>50%) in most mammalian membranes. Complex regulatory mechanisms control phosphatidylcholine biosynthesis (65, 137), which occurs by two pathways (Figure 1). In the first, choline is phosphorylated and then converted to cytidine diphosphocholine (CDP-choline). This intermediate, in combination with diacylglycerol, forms phosphatidylcholine and cytidine monophosphate. In the other pathway, phosphatidylethanolamine is sequentially methylated to form phosphatidylcholine, using S-adenosylmethionine as the methyl donor. The latter pathway is most active in liver, but has been identified in many other tissues, including brain and mammary gland (13, 138, 154). This pathway is discussed in detail below. Sphingomyelin, another important membrane phospholipid, is formed from phosphatidylcholine (47).
One of the functional consequences of dietary choline deficiency in humans was the development of fatty liver (hepatosteatosis) (17, 159) because a lack of phosphatidylcholine limits the export of excess triglyceride from liver in lipoproteins (155, 156). Also, choline deficiency in humans was associated with liver damage (elevated serum aminotransferases) (2, 4, 6, 159). Liver cells died by apoptosis when placed in choline-deficient medium (4, 61, 116), perhaps explaining why liver cells died and leaked enzymes into blood in choline-deficient humans. We now recognize that choline deficiency in humans can also present with muscle damage (33). This occurs because muscle membranes are more fragile (33) and perhaps because of induction of apoptosis via mechanisms similar to those described in liver (2, 54).
Human variation in dietary choline requirement is important for clinical practice in nutrition. Low plasma choline concentration occurs in up to 84% of patients who require total parenteral nutrition (TPN) (17–20, 113, 118), as does liver damage (elevated transaminases) (18, 126) and fatty liver (18). In some patients, the hepatic steatosis associated with TPN resolved with choline-supplementation and returned when standard TPN was reinstituted (18).
In 1998 the U.S. Institute of Medicine's Food and Nutrition Board established an adequate intake (AI) and tolerable upper limit (UL) for choline (57) (Table 1). The UL for choline was derived from the lowest observed adverse effect level (hypotension) in humans (57).
TABLE 1.
Dietary Reference Intake values for choline
| Population | Age | Adequate intake (AI) | Tolerable upper limit (UL) |
|---|---|---|---|
| AI for infants | 0 to 6 months | 125 mg/day, 18 mg/kg | Not possible to establish* |
| 6 to 12 months | 150 mg/day | ||
| AI for children | 1 through 3 years | 200 mg/day | 1000 mg/day |
| 4 through 8 years | 250 mg/day | 1000 mg/day | |
| 9 through 13 years | 375 mg/day | 2000 mg/day | |
| AI for males | 14 through 18 years | 550 mg/day | 3000 mg/day |
| 19 years and older | 550 mg/day | 3500 mg/day | |
| AI for females | 14 through 18 years | 400 mg/day | 3000 mg/day |
| 19 years and older | 425 mg/day | 3500 mg/day | |
| AI for pregnancy | All ages | 450 mg/day | Age-appropriate UL |
| AI for lactation | All ages | 550 mg/day | Age-appropriate UL |
Source of intake should be food and formula only.
From Institute of Medicine, National Academy of Sciences USA. 1998. Dietary Reference Intakes for Folate, Thiamin, Riboflavin, Niacin, Vitamin B12, Panthothenic Acid, Biotin, and Choline. Vol. 1. Washington, DC: Natl. Acad. Press.
CHOLINE, FOLATE, AND METHIONINE METABOLISM ARE INTERRELATED
Choline, methionine, and folate metabolism interact at the point that homocysteine is converted to methionine. Thus, any requirement for dietary choline must be considered in relation to these other nutrients. Homocysteine can be methylated to form methionine (38) by two parallel pathways, both of which lower homocysteine concentrations (98). In the first, vitamins B12 and folic acid are involved in a reaction catalyzed by methionine synthase (148). Deficiency of these nutrients (59, 114), or single nucleotide polymorphisms in the genes for the enzymes involved in this pathway (59, 145, 148), can result in elevated plasma homocysteine concentrations. In addition, tetrahydrofolate is needed to scavenge one-carbon groups when betaine is metabolized (92). The alternative pathway for the methylation of homocysteine to form methionine is catalyzed by betaine homocysteine methyltransferase (123), an enzyme whose activity has been reported to increase in rats during methionine excess (53). Betaine, derived from dietary choline by the action of choline dehydrogenase, is the methyl group donor in this reaction and supplemental oral betaine can lower plasma homocysteine concentrations (122, 150).
Perturbing metabolism of one of the methyl donors results in compensatory changes in the other methyl donors due to the intermingling of these metabolic pathways (66, 111, 140). Rats treated with the antifolate, methotrexate, had diminished pools of choline metabolites in liver (102, 111). Rats ingesting a choline-deficient diet had diminished tissue concentrations of methionine and S-adenosylmethionine (164) and doubled plasma homocysteine concentrations (139). We recently reported that humans who are depleted of choline have diminished capacity to methylate homocysteine and developed elevated homocysteine concentrations in plasma after a methionine loading test (34).
DIETARY INTAKE AND ENDOGENOUS SYNTHESIS OF CHOLINE
The U.S. Department of Agriculture recently developed the first database of choline content in foods (160, 162) (http://www.nal.usda.gov/fnic/foodcomp/Data/Choline/Choline.html). Choline is found in a wide variety of foods that contain membranes. Excellent sources of dietary choline include liver, eggs, and wheat germ. In foods, choline is found in free and esterified form (as phosphocholine, glycerophosphocholine, phosphatidylcholine, and sphingomyelin). Though it is likely that these forms are fungible, there is some evidence that they may have different bioavailability (23) because the lipid-soluble forms bypass the liver when absorbed from the diet, while the water-soluble forms enter the portal circulation and are mostly absorbed by the liver. Human milk is rich in choline compounds (55), and soy-derived infant formulas have lower total choline concentrations than do either human milk or bovine milk-derived formulas (55). Choline intake by humans on ad libitum diets for males and females averages 8.4 mg/kg and 6.7 mg/kg of choline per day, respectively (39). However, in a study of pregnant women in California, Shaw and colleagues observed intakes that were less than half this amount in 25% of the women studied (112).
The only source of choline other than diet is from the de novo biosynthesis of phosphatidylcholine catalyzed by PEMT. This enzyme uses S-adenosylmethionine as a methyl donor and forms a new choline moiety (13). When fed a diet deficient in choline, Pemt−/− mice developed fatty liver and severe liver damage and died; a choline-supplemented diet prevented this outcome (144) and reversed hepatic damage if begun early enough (143). The PEMT pathway is not just a minor pathway that backs up the cytidine diphosphocholine pathway for phosphatidylcholine biosynthesis. Pemt−/− mice have lower choline pools in liver despite being fed sufficient or supplemental amounts of dietary choline (166), suggesting that choline production by PEMT is a significant source of choline relative to dietary intake. When PEMT is deleted in mice, plasma homocysteine concentrations fall by 50%, and when it is overexpressed, plasma homocysteine concentrations increase by 40% (58, 115), demonstrating that PEMT activity is a major consumer of S-adenosylmethionine (and thereby a producer of homocysteine). In brains of Pemt−/− mice, there is enough excess S-adenosylmethionine that DNA is globally overmethylated (165).
CHOLINE REQUIREMENTS MAY VARY WITH GENDER
As noted earlier, premenopausal women, relative to males and postmenopausal women, are resistant to developing organ dysfunction when fed a low-choline diet. This probably is because premenopausal women have an enhanced capacity for de novo biosynthesis of choline moiety. Studies in animal models support this hypothesis, as female rats are less sensitive to choline deficiency than are male rats (130) and female mice produce more phosphatidylcholine via the PEMT pathway than do male mice (97). Estrogen status may be important for this increased PEMT activity (37); compared with controls, estradiol-treated castrated rats have more hepatic PEMT activity (157). Similar results were reported for diethylstilbestrol-treated roosters (142). Thus, estrogen could be the mediator of increased PEMT activity in women. During pregnancy, estradiol concentration rises from approximately 1 nM to 60 nM at term (1, 108), suggesting that capacity for endogenous synthesis of choline should be highest during the period when females need to support fetal development.
FEMALE CAPACITY FOR ENDOGENOUS SYNTHESIS OF CHOLINE MAY BE IMPORTANT
Pregnancy and lactation are times when demand for choline is especially high; transport of choline from mother to fetus (124, 125) depletes maternal plasma choline in humans (78). Thus, despite enhanced capacity to synthesize choline, the demand for this nutrient is so high that stores are depleted. Pregnant rats had diminished total liver choline compounds compared with nonmated controls and became as sensitive to choline-deficient diets as were male rats (161). Because milk contains a great deal of choline, lactation further increases maternal demand for choline, resulting in further depletion of tissue stores (55, 161). Pemt−/− mice abort pregnancies around 9−10 days gestation unless fed supplemental choline (personal observation; 165). These observations suggest that women depend on high rates of endogenous biosynthesis of choline induced by estrogen and dietary intake of choline to sustain normal pregnancy. It makes sense that evolution would develop mechanisms to assure that women are less susceptible to dietary choline deficiency and have adequate stores of choline prior to becoming pregnant. A better understanding of why there is variation in dietary choline requirements might be important for identifying women at greater risk for choline deficiency during pregnancy. This is a real possibility, as the data of Shaw and colleagues show, that women in the United States vary enough in dietary choline intake (from <300 mg/d to >500 mg/d) to influence the risk of having a baby with a birth defect (112). Choline nutriture during pregnancy is especially important because it influences brain development in the fetus (see below) and because it is important for maintaining normal plasma homocysteine concentrations during pregnancy (141). High maternal homocysteine concentrations are associated with increased incidence of birth defects (50).
THE FETUS LIVES IN A HIGH-CHOLINE ENVIRONMENT
Large amounts of choline are delivered to the fetus across the placenta, where choline transport systems pump it against a concentration gradient (124). It is interesting that the placenta is one of the few non-nervous tissues to store large amounts of choline as acetylcholine (70). Perhaps it is a special reserve storage pool that ensures delivery of choline to the fetus. In utero, the fetus is exposed to very high choline concentrations, with a progressive decline in blood choline concentration thereafter until adult levels are achieved after the first weeks of life (78). Choline concentration in amniotic fluid is tenfold greater than that present in maternal blood (S.H. Zeisel, unpublished observations). Plasma or serum choline concentrations are six- to sevenfold higher in the fetus and newborn than they are in the adult (99, 163). High levels of choline circulating in the neonate presumably ensure enhanced availability of choline to tissues. Neonatal rat brain efficiently extracts choline from blood (30), and increased serum choline in the neonatal rat is associated with twofold higher choline concentration in neonatal brain than is present later in life. Supplementing choline during the perinatal period further increases choline metabolite concentrations in blood and brain (43). There is a novel form of phosphatidylethanolamine-N-methyltransferase in neonatal rat brain that is extremely active (13); this isoform is not present in adult brain. Furthermore, in the brains of newborn rats, S-adenosylmethionine concentrations are 40−50 nmol/g of tissue (51)—levels probably sufficient to enable the neonatal form of phosphatidylethanolamine-N-methyltransferase to maintain high rates of activity. As previously mentioned, human and rat milk provide large amounts of choline to the neonate. The existence of these multiple mechanisms, which ensure the availability of choline to the fetus and neonate, suggest that evolutionary pressures favored exposure to high concentrations of choline in utero.
CHOLINE AND BRAIN DEVELOPMENT IN UTERO
It is widely accepted that folate affects embryogenesis of the brain. Currently, it is recommended that all women be folate supplemented during the periconception period because this reduces the risk for these serious defects in brain development (22, 57). Folic acid administered to women who had previously had a child with a neural tube defect lowered risk of recurrence by 72% (91). In rodents, choline is needed for normal neural tube closure in early pregnancy (40, 41); in humans, women in the lowest quartile for dietary choline intake had four times the risk (compared with women in the highest quartile) of having a baby with a neural tube defect (112). Choline and folate metabolism intersect at pathways for methyl-group donation (see discussion above) and it is reasonable to hypothesize that methylation reactions are the mechanism they share that influences neural tube closure. As discussed below, folate deficiency and choline deficiency have similar effects on stem cell proliferation and apoptosis in the brain (31, 32).
Choline and folate are also important in later periods of pregnancy when the memory center of brain (hippocampus) is developing. Maternal dietary choline supplementation or choline deficiency during late pregnancy in rodents was associated with significant and irreversible changes in hippocampal function in the adult rodent, including altered long-term potentiation (LTP) (63, 90, 103) and altered memory (79–84). More choline (about 4 times dietary levels) during days 11−17 of gestation in the rodent increased hippocampal progenitor cell proliferation (3, 5), decreased apoptosis in these cells (3, 5), enhanced LTP in the offspring when they were adult animals (63, 90, 103), and enhanced visuospatial and auditory memory by as much as 30% in the adult animals throughout their lifetimes (79–82, 84, 85, 152). Indeed, adult rodents decrement in memory as they age, and offspring exposed to extra choline in utero do not show this “senility” (81, 85). Mothers fed choline-deficient diets during late pregnancy have offspring with diminished progenitor cell proliferation and increased apoptosis in fetal hippocampus (3, 5), insensitivity to LTP when they were adult animals (63), and decremented visuospatial and auditory memory (84). The effects of perinatal choline supplementation on memory were initially found using radial-arm maze tasks and the Sprague-Dawley rat strain, but other laboratories have found similar results using other spatial memory tasks, such as the Morris water maze (14, 109), using passive avoidance paradigms (104), using measures of attention (88), using other strains of rats such as Long-Evans (127–129), and using mice (104). The effects of choline supplementation in utero were also detected in studies on effects of fetal alcohol exposure, where supplementation with choline attenuated behavioral alterations but not motor abnormalities (131, 132). Thus, in rodents choline supplementation during a critical period in pregnancy causes lifelong changes in brain structure and function.
The mechanism whereby a choline supplement supplied to the dams results in a permanent change in memory of their offspring has not been fully elucidated. Though the initial hypothesis was that the effect of neonatal choline supplementation on memory is mediated by increased brain choline with subsequent increased acetylcholine release, the amounts of choline that accumulate in fetal brain after treatment of the pregnant dam are not likely of sufficient magnitude to enhance acetylcholine release (43). Rather, supplementing choline to dams results in significantly greater accumulation of phosphocholine and betaine in fetal brain than in fetuses of controls (43).
The effects of choline on neural tube closure and on brain development could be mediated by changes in the expression of genes. Dietary choline deficiency not only depletes choline and choline metabolites in rats, but also decreases S-adenosylmethionine concentrations (117, 164), with resulting hypomethylation of DNA (74, 134). DNA methylation occurs at cytosine bases that are followed by a guanosine (CpG sites) (52) and influences many cellular events, including gene transcription, genomic imprinting, and genomic stability (60, 64, 105). In mammals, about 60% to 90% of 5′-CpG-3′ islands are methylated (62). When this modification occurs in promoter regions, gene expression is altered (9); increased methylation is associated with gene silencing or reduced gene expression (62). In choline-deficient cells in culture, and in fetal rodent brains from mothers fed choline-deficient diets, methylation of the CDKN3 gene promoter is decreased, resulting in overexpression of this gene, which inhibits cell proliferation (93, 94). In choline-deficient liver, there is hypomethylation of specific CCGG sites within several genes for which mRNA levels were increased, including c-myc, c-fos, and c-Ha-ras (24). Hypomethylation of CpG sites and c-myc gene overexpression occurs in hepatocellular carcinomas induced by a choline-deficient diet in rats (134). It is also reasonable that maternal diet during pregnancy could alter the methylation status of fetal DNA. For example, feeding pregnant pseudoagouti Avy/a mouse dams a choline methyl-supplemented diet altered epigenetic regulation of agouti expression in their offspring, as indicated by increased agouti/black mottling of their coats (28, 153). It is clear that the dietary manipulation of methyl donors (either deficiency or supplementation) can have a profound impact upon gene expression and, by consequence, upon the homeostatic mechanisms that ensure the normal function of physiological processes.
Whether these findings in rodents apply as well to humans is not known. Of course, human and rat brains mature at different rates, with rat brain comparatively more mature at birth than is the human brain. In humans, the architecture of the hippocampus continues to develop after birth, and by 4 years of age it closely resembles adult structure (35). This area of brain is one of the few areas in which nerve cells continue to multiply slowly throughout life (76, 136). Are we varying the availability of choline when we feed infants formula instead of milk? Does the form and amount of choline ingested contribute to variations in memory observed between humans? All are good questions that are worthy of additional research. The observation by Shaw and colleagues (112) that women eating low-choline diets have a greatly increased risk for having a baby with a neural tube defect supports the suggestion that the basic research in rodents will be applicable to the human condition.
CHOLINE AND NEURONAL FUNCTION IN ADULTS
As discussed above, choline accelerates the synthesis and release of acetylcholine in nerve cells (12, 26, 27, 49, 133, 135). A specific carrier mechanism transports free choline across the blood-brain barrier at a rate that is proportional to serum choline concentration (29, 100). Phosphatidylcholine may be carried into neurons as part of an ApoE lipoprotein (101, 149). Choline derived from phosphatidylcholine may be especially important when extracellular choline is in short supply, as might be expected to occur in advanced age because of decreased brain choline uptake in older adults (25). Abnormal phospholipid metabolism in Alzheimer's disease (96) results in reduced levels in brain (at autopsy) of phosphatidylcholine, phosphatidylethanolamine, choline, and ethanolamine and increased levels of glycerophosphocholine and glycerophosphoethanolamine.
Mice and rats exhibit an age-related loss of memory function. In adult animals, chronic dietary intake low in choline exacerbated this memory loss, whereas choline-enriched diets eaten by adult animals diminished memory loss (8). Young choline-deficient mice performed as poorly as much older mice; choline-supplemented older mice performed as well as young three-month-old mice. Supplemented mice did have changes in the shape (more dendritic spines) of their neurons in the hippocampus (87). No equivalent experiments have been reported in humans.
Studies have been performed on the effects of short-term administration of choline or lecithin on the memory of normal humans and the results reported vary. In a double-blind study using normal college students, 25 g of phosphatidylcholine caused significant improvement in explicit memory, as measured by a serial learning task; this improvement might have been due to the improved responses of slow learners (69). A single oral 10 g dose of choline chloride given to normal volunteers significantly decreased the number of trials needed to master a serial-learning word test (119). The precursor for formation of phosphatidylcholine, cytidine diphosphocholine (CDP-choline), also has been tested for memory-enhancing effects. In a randomized, double-blind, placebo-controlled study, volunteers were treated with 1000 mg/d CDP-choline or placebo for three months. CDP-choline improved immediate and delayed logical memory (121). In a second study, oral administration of CDP-choline (500−1000 mg/day) for four weeks to elderly subjects with memory deficits but without dementia resulted in improved memory in free recall tasks, but not in recognition tests (7). In a double-blind study, patients with early Alzheimer-type dementia were treated with 25 g/day phosphatidylcholine for six months. Modest improvements were observed compared with placebo in several memory tests (71, 73). However, there are studies in which the choline effect on memory was not observed in normal subjects (36, 48, 89) or in patients with dementias (15, 42, 147).
GENE POLYMORPHISMS AND DIETARY CHOLINE REQUIREMENTS
As discussed above, there is a difference in the dietary choline requirement for premenopausal women versus postmenopausal women and men. Estrogen induction of endogenous synthesis is the likely reason. However, additional reasons for this variance in dietary choline requirements must be factors because some men and women develop organ dysfunction when fed a low-choline diet, while others do not. Some men and women require more than 850 mg/70 kg/d choline in their diet, while others require less than 550 mg/kg/d (S.H. Zeisel, unpublished data).
Genetic variation likely underlies these differences in dietary requirements. From what we understand about choline metabolism, several pathways could influence how much choline is required from diet, and single-nucleotide polymorphisms (SNPs) in these pathways might be of importance. Specifically, polymorphisms in the folate pathways could limit the availability of methyltetrahydrofolate and thereby increase use of choline as a methyl donor. Polymorphisms in the PEMT gene might alter endogenous synthesis of choline, and polymorphisms in other genes of choline metabolism could influence dietary requirements by changing the utilization of choline moiety.
We developed a clinical methodology for phenotyping individuals with respect to their susceptibility to developing organ dysfunction when fed a low-choline diet (21, 33, 34). In a 95-day repeated-measure, within-subject study with graded repletion, adult men and women (pre- and postmenopausal) ages 18−70 were fed a standard diet containing a known amount of choline (550 mg/70 kg/d; baseline). On day 11 subjects were placed on a diet containing <50 mg choline/day for up to 42 days. Blood and urine were collected to measure various experimental parameters of dietary choline status and markers of organ dysfunction (serum creatine phosphokinase, alanine amino transferase, aspartate amino transferase) and liver fat level was assessed by magnetic resonance imaging. If at some point during the depletion period, functional markers indicated organ dysfunction associated with choline deficiency, subjects were switched to a diet containing 137 mg/70 kg/d choline (low or normal folate) for 10 days. If the functional markers indicated that deficiency status persisted, subjects were then switched to a diet containing 275 mg/70 kg/d choline (low or normal folate) for 10 days. If markers of organ dysfunction did not indicate repletion at this time, subjects were switched to a diet containing 413 mg/70 kg/d choline (low or normal folate) for 10 days. If still not repleted, subjects were fed a diet containing at least 550 mg/70 kg/d choline (low or normal folate) until repleted.
We found that premenopausal women were less likely to develop organ dysfunction compared with men and postmenopausal women when challenged with a choline-deficient diet (L.M. Fischer, K.A. da Costa, L. Kwock, P. Stewart, S. Stabler, R. Allen, S.H. Zeisel, manuscript in preparation). We found no effect of folate status on susceptibility to choline deficiency.
We examined whether major genetic variants of folate metabolism modify susceptibility of humans to choline deficiency (67). Premenopausal women who were carriers of the very common 5,10-methylenetetrahydrofolate dehydrogenase-1958A (MTHFD1) gene allele were more than 15 times as likely as noncarriers to develop signs of choline deficiency (p < 0.0001) on the low-choline diet. Sixty-three percent of our study population had at least one allele for this SNP. As noted above, two additional reactions, mediated by methylenetetrahydrofolate dehydrogenase and methenyltetrahydrofolate cyclohydrolase, can convert 10-formyltetrahydrofolate to 5,10-methylenetetrahydrofolate (Figure 1). Whereas the formation of 5-methyltetrahydrofolate is practically irreversible in vivo, the interconversion of 5,10-methylenetetrahydrofolate and 10-formyltetrahydrofolate is closer to equilibrium (56). This means that 5,10-methylenetetrahydrofolate may be directed either toward homocysteine remethylation or away from it. The MTHFD1 G1958A polymorphism may thus affect the delicately balanced flux between 5,10-methylenetetrahydrofolate and 10-formyltetrahydrofolate and thereby influence the availability of 5-methyl tetrahydrofolate for homocysteine remethylation. This would increase demand for choline as a methyl group donor. It is of interest that the risk of having a child with a neural tube defect increases in mothers with the G1958A SNP in MTHFD1 (16).
5,10-methylenetetrahydrofolate can be reduced by MTHFR to 5-methyltetrahydrofolate. Mice that are Mthfr−/− become choline deficient (110), an important observation because many humans have genetic polymorphisms that alter the activity of this enzyme (106, 151). In our study in humans described above, the effects of the C677T and A1298C polymorphisms of the MTHFR gene and the A80C polymorphism of the reduced folate carrier gene were not statistically significant. A larger study is needed to have adequate power to determine if these SNPs have some effect on human choline requirements.
As noted above, PEMT encodes for a protein responsible for endogenous formation of choline. We examined genes of choline metabolism and identified an SNP in the promoter region of the PEMT gene (−939G → C; rs12325817) that was associated with greatly increased susceptibility to choline deficiency in women (K.A. da Costa, O.G. Kozyreva, J. Song, J.A. Galank, L.M. Fischer, S.H. Zeisel, manuscript submitted). Given the sexual differences in the effect of PEMT rs12325817, it is possible that this SNP alters the estrogen responsiveness of the promoter. The frequency of this variant allele was 0.74. Another SNP in exon 8 of PEMT results in a 30% loss of function and is associated with increased risk for nonalcoholic fatty liver disease (120). Studies are under way in our laboratory to identify other genetic differences that contribute to individual variability in dietary requirements for choline.
SUMMARY
Understanding dietary choline requirement and its modulation by genetic polymorphisms has public health significance, especially because this nutrient is important for brain development. Current dietary recommendations for choline are likely too low for some men. In addition, these recommendations did not consider genetic variation as a modulator of dietary requirement because it was assumed that functional polymorphisms would be too rare (<5% of population) to be considered. It is clear that this assumption is not true for SNPs in folate metabolism (where 63% of subjects had at least one allele for the MTHFD1 SNP) or for SNPs in choline metabolism (where 74% of subjects had at least one allele for the PEMT promoter SNP). As discussed above, women with low dietary choline intake have a markedly increased risk of having a baby with neural tube defects (112). There is solid science in animal models that suggests choline is critical for normal brain development (31). Choline deficiency has other health consequences—it is associated with liver and muscle damage and with an exaggerated plasma homocysteine rise after a methionine load (34). Elevated plasma homocysteine is an independent risk factor for cardiovascular disease and stroke in humans (44, 45, 77).
ACKNOWLEDGMENTS
This work was funded by a grant from the National Institutes of Health (DK55865, AG09525, ES012997) and the U.S. Department of Agriculture (2004-01,833). Support for this work was also provided by grants from the NIH to the UNC Clinical Nutrition Research Unit (DK56350), the UNC General Clinical Research Center (RR00046), and the Center for Environmental Health and Susceptibility (ES10126).
LITERATURE CITED
- 1.Adeyemo O, Jeyakumar H. Plasma progesterone, estradiol-17 beta and testosterone in maternal and cord blood, and maternal human chorionic gonadotropin at parturition. Afr. J. Med. Med. Sci. 1993;22:55–60. [PubMed] [Google Scholar]
- 2.Albright CD, da Costa KA, Craciunescu CN, Klem E, Mar MH, Zeisel SH. Regulation of choline deficiency apoptosis by epidermal growth factor in CWSV-1 rat hepatocytes. Cell Physiol. Biochem. 2005;15:59–68. doi: 10.1159/000083653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res. 1999;115:123–29. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 4.Albright CD, Lui R, Bethea TC, da Costa K-A, Salganik RI, Zeisel SH. Choline deficiency induces apoptosis in SV40-immortalized CWSV-1 rat hepatocytes in culture. FASEB J. 1996;10:510–16. doi: 10.1096/fasebj.10.4.8647350. [DOI] [PubMed] [Google Scholar]
- 5.Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res. 1999;113:13–20. doi: 10.1016/s0165-3806(98)00183-7. [DOI] [PubMed] [Google Scholar]
- 6.Albright CD, Zeisel SH. Choline deficiency causes increased localization of TGFβ1 signaling proteins and apoptosis in rat liver. Pathobiology. 1997;65:264–70. doi: 10.1159/000164137. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez XA, Laredo M, Corzo D, Fernandez-Novoa L, Mouzo R, et al. Citicoline improves memory performance in elderly subjects. Methods Find. Exp. Clin. Pharmacol. 1997;19:201–10. [PubMed] [Google Scholar]
- 8.Bartus RT, Dean RL, Goas JA, Lippa AS. Age-related changes in passive avoidance retention: modulation with dietary choline. Science. 1980;209:301–3. doi: 10.1126/science.7384805. [DOI] [PubMed] [Google Scholar]
- 9.Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–13. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 10.Blusztajn JK, Holbrook PG, Lakher M, Liscovitch M, Maire JC, et al. “Autocannibalism” of membrane cholinephospholipids: physiology and pathology. Psychopharmacol. Bull. 1986;22:781–86. [PubMed] [Google Scholar]
- 11.Blusztajn JK, Liscovitch M, Richardson UI. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc. Natl. Acad. Sci. USA. 1987;84:5474–77. doi: 10.1073/pnas.84.15.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science. 1983;221:614–20. doi: 10.1126/science.6867732. [DOI] [PubMed] [Google Scholar]
- 13.Blusztajn JK, Zeisel SH, Wurtman RJ. Developmental changes in the activity of phosphatidylethanolamine N-methyltransferases in rat brain. Biochem. J. 1985;232:505–11. doi: 10.1042/bj2320505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandner C. Perinatal choline treatment modifies the effects of a visuo-spatial attractive cue upon spatial memory in naive adult rats. Brain. Res. 2002;928:85–95. doi: 10.1016/s0006-8993(01)03363-7. [DOI] [PubMed] [Google Scholar]
- 15.Brinkman SD, Smith RC, Meyer JS, Vroulis G, Shaw T, et al. Lecithin and memory training in suspected Alzheimer's disease. J. Gerontol. 1982;37:4–9. doi: 10.1093/geronj/37.1.4. [DOI] [PubMed] [Google Scholar]
- 16.Brody LC, Conley M, Cox C, Kirke PN, McKeever MP, et al. A polymorphism, R653Q, in the trifunctional enzyme methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase/formyltetrahydrofolate synthetase is a maternal genetic risk factor for neural tube defects: report of the Birth Defects Research Group. Am. J. Hum. Genet. 2002;71:1207–15. doi: 10.1086/344213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchman A, Dubin M, Moukarzel A, Jenden D, Roch M, et al. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995;22:1399–403. [PubMed] [Google Scholar]
- 18.Buchman AL, Ament ME, Sohel M, Dubin M, Jenden DJ, et al. Choline deficiency causes reversible hepatic abnormalities in patients receiving parenteral nutrition: proof of a human choline requirement: a placebo-controlled trial. J. Parenter. Enteral Nutr. 2001;25:260–68. doi: 10.1177/0148607101025005260. [DOI] [PubMed] [Google Scholar]
- 19.Buchman AL, Moukarzel A, Jenden DJ, Roch M, Rice K, Ament ME. Low plasma free choline is prevalent in patients receiving long term parenteral nutrition and is associated with hepatic aminotransferase abnormalities. Clin. Nutr. 1993;12:33–37. doi: 10.1016/0261-5614(93)90143-r. [DOI] [PubMed] [Google Scholar]
- 20.Burt ME, Hanin I, Brennan MF. Choline deficiency associated with total parenteral nutrition. Lancet. 1980;2:638–39. doi: 10.1016/s0140-6736(80)90301-3. [DOI] [PubMed] [Google Scholar]
- 21.Busby MG, Fischer L, Da Costa KA, Thompson D, Mar MH, Zeisel SH. Choline- and betaine-defined diets for use in clinical research and for the management of trimethylaminuria. J. Am. Diet. Assoc. 2004;104:1836–45. doi: 10.1016/j.jada.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. Morbid. Mortal. Wkly. Rep. 1992;41:1–7. [PubMed] [Google Scholar]
- 23.Cheng W-L, Holmes-McNary MQ, Mar M-H, Lien EL, Zeisel SH. Bioavail-ability of choline and choline esters from milk in rat pups. J. Nutr. Biochem. 1996;7:457–64. [Google Scholar]
- 24.Christman JK, Sheikhnejad G, Dizik M, Abileah S, Wainfan E. Reversibility of changes in nucleic acid methylation and gene expression induced in rat liver by severe dietary methyl deficiency. Carcinogenesis. 1993;14:551–57. doi: 10.1093/carcin/14.4.551. [DOI] [PubMed] [Google Scholar]
- 25.Cohen BM, Renshaw PF, Stoll AL, Wurtman RJ, Yurgelun-Todd D, Babb SM. Decreased brain choline up-take in older adults. An in vivo proton magnetic resonance spectroscopy study. JAMA. 1995;274:902–7. [PubMed] [Google Scholar]
- 26.Cohen EL, Wurtman RJ. Brain acetylcholine: increase after systemic choline administration. Life Sci. 1975;16:1095–102. doi: 10.1016/0024-3205(75)90194-0. [DOI] [PubMed] [Google Scholar]
- 27.Cohen EL, Wurtman RJ. Brain acetylcholine: control by dietary choline. Science. 1976;191:561–62. doi: 10.1126/science.1251187. [DOI] [PubMed] [Google Scholar]
- 28.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J. Nutr. 2002;132:2393–400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- 29.Cornford EM, Braun LD, Oldendorf WH. Carrier mediated blood-brain barrier transport of choline and certain choline analogs. J. Neurochem. 1978;30:299–308. doi: 10.1111/j.1471-4159.1978.tb06530.x. [DOI] [PubMed] [Google Scholar]
- 30.Cornford EM, Cornford ME. Nutrient transport and the blood-brain barrier in developing animals. Fed. Proc. 1986;45:2065–72. [PubMed] [Google Scholar]
- 31.Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J. Nutr. 2003;133:3614–18. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craciunescu CN, Brown EC, Mar MH, Albright CD, Nadeau MR, Zeisel SH. Folic acid deficiency during late gestation decreases progenitor cell proliferation and increases apoptosis in fetal mouse brain. J. Nutr. 2004;134:162–66. doi: 10.1093/jn/134.1.162. [DOI] [PubMed] [Google Scholar]
- 33.da Costa KA, Badea M, Fischer LM, Zeisel SH. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am. J. Clin. Nutr. 2004;80:163–70. doi: 10.1093/ajcn/80.1.163. [DOI] [PubMed] [Google Scholar]
- 34.da Costa KA, Gaffney CE, Fischer LM, Zeisel SH. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am. J. Clin. Nutr. 2005;81:440–44. doi: 10.1093/ajcn.81.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dani S, Hori A, Walter G, editors. Principals of Neural Aging. Elsevier; Amsterdam: 1997. [Google Scholar]
- 36.Drachman DA, Glosser G, Fleming P, Longenecker G. Memory decline in the aged: treatment with lecithin and physostigmine. Neurology. 1982;32:944–50. doi: 10.1212/wnl.32.9.944. [DOI] [PubMed] [Google Scholar]
- 37.Drouva SV, LaPlante E, Leblanc P, Bechet JJ, Clauser H, Kordon C. Estradiol activates methylating enzyme(s) involved in the conversion of phosphatidylethanolamine to phosphatidylcholine in rat pituitary membranes. Endocrinology. 1986;119:2611–22. doi: 10.1210/endo-119-6-2611. [DOI] [PubMed] [Google Scholar]
- 38.Finkelstein JD. Pathways and regulation of homocysteine metabolism in mammals. Semin. Thromb. Hemost. 2000;26:219–25. doi: 10.1055/s-2000-8466. [DOI] [PubMed] [Google Scholar]
- 39.Fischer LM, Scearce JA, Mar MH, Patel JR, Blanchard RT, et al. Ad libitum choline intake in healthy individuals meets or exceeds the proposed adequate intake level. J. Nutr. 2005;135:826–29. doi: 10.1093/jn/135.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher MC, Zeisel SH, Mar MH, Sadler TW. Inhibitors of choline up-take and metabolism cause developmental abnormalities in neurulating mouse embryos. Teratology. 2001;64:114–22. doi: 10.1002/tera.1053. [DOI] [PubMed] [Google Scholar]
- 41.Fisher MC, Zeisel SH, Mar MH, Sadler TW. Perturbations in choline metabolism cause neural tube defects in mouse embryos in vitro. FASEB J. 2002;16:619–21. doi: 10.1096/fj.01-0564fje. [DOI] [PubMed] [Google Scholar]
- 42.Fitten LJ, Perryman KM, Gross PL, Fine H, Cummins J, Marshall C. Treatment of Alzheimer's disease with short-and long-term oral THA and lecithin: a double-blind study. Am. J. Psychiatry. 1990;147:239–42. doi: 10.1176/ajp.147.2.239. [DOI] [PubMed] [Google Scholar]
- 43.Garner SC, Mar M-H, Zeisel SH. Choline distribution and metabolism in pregnant rats and fetuses are influenced by the choline content of the maternal diet. J. Nutr. 1995;125:2851–58. doi: 10.1093/jn/125.11.2851. [DOI] [PubMed] [Google Scholar]
- 44.Glueck CJ, Shaw P, Lang JE, Tracy T, Sieve-Smith L, Wang Y. Evidence that homocysteine is an independent risk factor for atherosclerosis in hyperlipidemic patients. Am. J. Cardiol. 1995;75:132–36. doi: 10.1016/s0002-9149(00)80061-2. [DOI] [PubMed] [Google Scholar]
- 45.Goddijn-Wessel T, Wouters M, van de Molen E, Spuijbroek M, Steegers-Theunissen R, et al. Hyperhomocysteinemia: a risk factor for placental abruption or infarction. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996;66:23–29. doi: 10.1016/0301-2115(96)02383-4. [DOI] [PubMed] [Google Scholar]
- 46.Guba S, Fink L, Fonseca V. Hyperhomocysteinemia. An emerging and important risk factor for thromboembolic and cardiovascular disease. Am. J. Clin. Pathol. 1996;106:709–22. doi: 10.1093/ajcp/106.6.709. [DOI] [PubMed] [Google Scholar]
- 47.Hanada K, Horii M, Akamatsu Y. Functional reconstitution of sphingomyelin synthase in Chinese hamster ovary cell membranes. Biochim. Biophys. Acta. 1991;1086:151–56. doi: 10.1016/0005-2760(91)90002-y. [DOI] [PubMed] [Google Scholar]
- 48.Harris CM, Dysken MW, Fovall P, Davis JM. Effect of lecithin on memory in normal adults. Am. J. Psychiatry. 1983;140:1010–12. doi: 10.1176/ajp.140.8.1010. [DOI] [PubMed] [Google Scholar]
- 49.Haubrich DR, Wang PF, Clody DE, Wedeking PW. Increase in rat brain acetylcholine induced by choline or deanol. Life Sci. 1975;17:975–80. doi: 10.1016/0024-3205(75)90451-8. [DOI] [PubMed] [Google Scholar]
- 50.Hobbs CA, Cleves MA, Melnyk S, Zhao W, James SJ. Congenital heart defects and abnormal maternal biomarkers of methionine and homocysteine metabolism. Am. J. Clin. Nutr. 2005;81:147–53. doi: 10.1093/ajcn/81.1.147. [DOI] [PubMed] [Google Scholar]
- 51.Hoffman DR, Cornatzer WE, Duerre JA. Relationship between tissue levels of S-adenosylmethionine, S-adenosylhomocysteine, and transmethylation reactions. Can. J. Biochem. 1979;57:56–65. doi: 10.1139/o79-007. [DOI] [PubMed] [Google Scholar]
- 52.Holliday R, Grigg GW. DNA methylation and mutation. Mutat. Res. 1993;285:61–67. doi: 10.1016/0027-5107(93)90052-h. [DOI] [PubMed] [Google Scholar]
- 53.Holm PI, Bleie O, Ueland PM, Lien EA, Refsum H, et al. Betaine as a determinant of postmethionine load total plasma homocysteine before and after B-vitamin supplementation. Arterioscler. Thromb. Vasc. Biol. 2004;24:301–7. doi: 10.1161/01.ATV.0000114569.54976.31. [DOI] [PubMed] [Google Scholar]
- 54.Holmes-McNary M, Baldwin J, Zeisel SH. Opposing regulation of choline deficiency-induced apoptosis by p53 and NF-κB. J. Biol. Chem. 2001;276:41197–204. doi: 10.1074/jbc.M010936200. [DOI] [PubMed] [Google Scholar]
- 55.Holmes-McNary M, Cheng WL, Mar MH, Fussell S, Zeisel SH. Choline and choline esters in human and rat milk and infant formulas. Am. J. Clin. Nutr. 1996;64:572–76. doi: 10.1093/ajcn/64.4.572. [DOI] [PubMed] [Google Scholar]
- 56.Horne DW. Neither methionine nor nitrous oxide inactivation of methionine synthase affect the concentration of 5,10-methylenetetrahydrofolate in rat liver. J. Nutr. 2003;133:476–78. doi: 10.1093/jn/133.2.476. [DOI] [PubMed] [Google Scholar]
- 57.Inst. Med., Natl. Acad. Sci. USA . Dietary Reference Intakes for Folate, Thiamin, Riboflavin, Niacin, Vita-min B12, Panthothenic Acid, Biotin, and Choline. Natl. Acad. Press; Washington, DC: 1998. Choline. pp. 390–422. [PubMed] [Google Scholar]
- 58.Jacobs RL, Stead LM, Devlin C, Tabas I, Brosnan ME, et al. Physiological regulation of phospholipid methylation alters plasma homocysteine in mice. J. Biol. Chem. 2005;280:28299–305. doi: 10.1074/jbc.M501971200. [DOI] [PubMed] [Google Scholar]
- 59.Jacques PF, Bostom AG, Wilson PW, Rich S, Rosenberg IH, Selhub J. Determinants of plasma total homocysteine concentration in the Framingham Off-spring cohort. Am. J. Clin. Nutr. 2001;73:613–21. doi: 10.1093/ajcn/73.3.613. [DOI] [PubMed] [Google Scholar]
- 60.Jaenisch R. DNA methylation and imprinting: Why bother? Trends Genet. 1997;13:323–29. doi: 10.1016/s0168-9525(97)01180-3. [DOI] [PubMed] [Google Scholar]
- 61.James SJ, Miller BJ, Basnakian AG, Pogribny IP, Pogribna M, Muskhelishvili L. Apoptosis and proliferation under conditions of deoxynucleotide pool imbalance in liver of folate/methyl deficient rats. Carcinogenesis. 1997;18:287–93. doi: 10.1093/carcin/18.2.287. [DOI] [PubMed] [Google Scholar]
- 62.Jeltsch A. Beyond Watson and Crick: DNA methylation and molecular enzymology of DNA methyltransferases. Chembiochem. 2002;3:382. doi: 10.1002/1439-7633(20020402)3:4<274::AID-CBIC274>3.0.CO;2-S. (Erratum) [DOI] [PubMed] [Google Scholar]
- 63.Jones JP, Meck W, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Brain Res. Dev. Brain Res. 1999;118:159–67. doi: 10.1016/s0165-3806(99)00103-0. [DOI] [PubMed] [Google Scholar]
- 64.Jones PA, Gonzalgo ML. Altered DNA methylation and genome instability: a new pathway to cancer? Proc. Natl. Acad. Sci. USA. 1997;94:2103–5. doi: 10.1073/pnas.94.6.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kent C. Regulation of phosphatidylcholine biosynthesis. Prog. Lipid Res. 1990;29:87–105. doi: 10.1016/0163-7827(90)90010-i. [DOI] [PubMed] [Google Scholar]
- 66.Kim Y-I, Miller JW, da Costa K-A, Nadeau M, Smith D, et al. Folate deficiency causes secondary depletion of choline and phosphocholine in liver. J. Nutr. 1995;124:2197–203. doi: 10.1093/jn/124.11.2197. [DOI] [PubMed] [Google Scholar]
- 67.Kohlmeier M, da Costa KA, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc. Natl. Acad. Sci. USA. 2005;102:16025–30. doi: 10.1073/pnas.0504285102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuksis A, Mookerjea S. Choline. Nutr. Rev. 1978;36:201–7. doi: 10.1111/j.1753-4887.1978.tb07359.x. [DOI] [PubMed] [Google Scholar]
- 69.Ladd SL, Sommer SA, LaBerge S, Toscano W. Effect of phosphatidylcholine on explicit memory. Clin. Neuropharm. 1993;16:540–49. [PubMed] [Google Scholar]
- 70.Leventer SM, Rowell PP. Investigation of the rate-limiting step in the synthesis of acetylcholine by the human placenta. Placenta. 1984;5:261–70. doi: 10.1016/s0143-4004(84)80036-3. [DOI] [PubMed] [Google Scholar]
- 71.Levy R. Lecithin in Alzheimer's disease. Lancet. 1982;2:671–72. doi: 10.1016/s0140-6736(82)92777-5. [DOI] [PubMed] [Google Scholar]
- 72.Lin CS, Wu RD. Choline oxidation and choline dehydrogenase. J. Prot. Chem. 1986;5:193–200. [Google Scholar]
- 73.Little A, Levy R, Chuaqui-Kidd P, Hand D. A double-blind, placebo controlled trial of high-dose lecithin in Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 1985;48:736–42. doi: 10.1136/jnnp.48.8.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Locker J, Reddy TV, Lombardi B. DNA methylation and hepatocarcinogenesis in rats fed a choline devoid diet. Carcinogenesis. 1986;7:1309–12. doi: 10.1093/carcin/7.8.1309. [DOI] [PubMed] [Google Scholar]
- 75.Loy R, Heyer D, Williams CL, Meck WH. Choline-induced spatial memory facilitation correlates with altered distribution and morphology of septal neurons. Adv. Exp. Med. Biol. 1991;295:373–82. doi: 10.1007/978-1-4757-0145-6_21. [DOI] [PubMed] [Google Scholar]
- 76.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J. Comp. Neurol. 1999;406:449–60. [PubMed] [Google Scholar]
- 77.McCully K. Homocysteine and vascular disease. Nat. Med. 1996;2:386–89. doi: 10.1038/nm0496-386. [DOI] [PubMed] [Google Scholar]
- 78.McMahon KE, Farrell PM. Measurement of free choline concentrations in maternal and neonatal blood by micropyrolysis gas chromatography. Clin. Chim. Acta. 1985;149:1–12. doi: 10.1016/0009-8981(85)90267-0. [DOI] [PubMed] [Google Scholar]
- 79.Meck W, Williams C. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport. 1997;8:2831–35. doi: 10.1097/00001756-199709080-00005. [DOI] [PubMed] [Google Scholar]
- 80.Meck W, Williams C. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport. 1997;8:3053–59. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- 81.Meck W, Williams C. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. Neuroreport. 1997;8:3045–51. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- 82.Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev. Psychobiol. 1988;21:339–53. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- 83.Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav. Neurosci. 1989;103:1234–41. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- 84.Meck WH, Williams CL. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Brain Res. Dev. Brain Res. 1999;118:51–59. doi: 10.1016/s0165-3806(99)00105-4. [DOI] [PubMed] [Google Scholar]
- 85.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci. Biobehav. Rev. 2003;27:385–99. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 86.Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB J. 2004;18:545–47. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- 87.Mervis RF. Chronic dietary choline represses age-related loss of dendritic spines in mouse neocortical pyramidal cells. J. Neuropathol. Exp. Neurol. 1982;41:363. [Google Scholar]
- 88.Mohler E, Meck W, Williams C. Sustained attention in adult mice is modulated by prenatal choline availability. Int. J. Comp. Psychol. 2001;14:136–50. [Google Scholar]
- 89.Mohs RC, Davis KL. Choline chlo-ride effects on memory: correlation with the effects of physostigmine. Psychiatry Res. 1980;2:149–56. doi: 10.1016/0165-1781(80)90071-2. [DOI] [PubMed] [Google Scholar]
- 90.Montoya DA, White AM, Williams CL, Blusztajn JK, Meck WH, Swartzwelder HS. Prenatal choline exposure alters hippocampal responsiveness to cholinergic stimulation in adulthood. Brain Res. Dev. Brain Res. 2000;123:25–32. doi: 10.1016/s0165-3806(00)00075-4. [DOI] [PubMed] [Google Scholar]
- 91.MRC Vitamin Study Res. Group Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–37. [PubMed] [Google Scholar]
- 92.Mudd SH, Ebert MH, Scriver CR. Labile methyl group balances in the human: the role of sarcosine. Metabolism. 1980;29:707–20. doi: 10.1016/0026-0495(80)90192-4. [DOI] [PubMed] [Google Scholar]
- 93.Niculescu MD, Craciunescu CN, Zeisel SH. Gene expression profiling of choline-deprived neural precursor cells isolated from mouse brain. Brain Res. Mol. Brain Res. 2005;134:309–22. doi: 10.1016/j.molbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 94.Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J. Neurochem. 2004;89:1252–59. doi: 10.1111/j.1471-4159.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methio-nine and choline. J. Nutr. 2002;132:2333–35S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- 96.Nitsch RM, Blusztajn JK, Pittas AG, Slack BE, Growdon JH, Wurtman RJ. Evidence for a membrane defect in Alzheimer disease brain. Proc. Natl. Acad. Sci. USA. 1992;89:1671–75. doi: 10.1073/pnas.89.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Noga AA, Vance DE. A gender-specific role for phosphatidylethanolamine N-methyltransferase-derived phosphatidylcholine in the regulation of plasma high density and very low density lipoproteins in mice. J. Biol. Chem. 2003;278:21851–59. doi: 10.1074/jbc.M301982200. [DOI] [PubMed] [Google Scholar]
- 98.Olthof MR, van Vliet T, Boelsma E, Verhoef P. Low dose betaine supplementation leads to immediate and long term lowering of plasma homocysteine in healthy men and women. J. Nutr. 2003;133:4135–38. doi: 10.1093/jn/133.12.4135. [DOI] [PubMed] [Google Scholar]
- 99.Ozarda IY, Uncu G, Ulus IH. Free and phospholipid-bound choline concentrations in serum during pregnancy, after delivery and in newborns. Arch. Physiol. Biochem. 2002;110:393–99. doi: 10.1076/apab.110.5.393.11832. [DOI] [PubMed] [Google Scholar]
- 100.Pardridge WM. Blood-brain transport of nutrients. Introduction. Fed. Proceed. 1986;45:2047–49. [PubMed] [Google Scholar]
- 101.Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci. 1994;17:525–30. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 102.Pomfret EA, da Costa K, Zeisel SH. Effects of choline deficiency and methotrexate treatment upon rat liver. J. Nutr. Biochem. 1990;1:533–41. doi: 10.1016/0955-2863(90)90039-n. [DOI] [PubMed] [Google Scholar]
- 103.Pyapali G, Turner D, Williams C, Meck W, Swartzwelder HS. Prenatal choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J. Neurophysiol. 1998;79:1790–96. doi: 10.1152/jn.1998.79.4.1790. [DOI] [PubMed] [Google Scholar]
- 104.Ricceri L, Berger-Sweeney J. Postnatal choline supplementation in preweanling mice: sexually dimorphic behavioral and neurochemical effects. Behav. Neurosci. 1998;112:1387–92. [PubMed] [Google Scholar]
- 105.Robertson KD, Wolffe AP. DNA methylation in health and disease. Nat. Rev. Genet. 2000;1:11–19. doi: 10.1038/35049533. [DOI] [PubMed] [Google Scholar]
- 106.Rozen R. Molecular genetic aspects of hyperhomocysteinemia and its relation to folic acid. Clin. Invest. Med. 1996;19:171–78. [PubMed] [Google Scholar]
- 107.Saito S, Iida A, Sekine A, Miura Y, Sakamoto T, et al. Identification of 197 genetic variations in six human methyltransferase genes in the Japanese population. J. Hum. Genet. 2001;46:529–37. doi: 10.1007/s100380170035. [DOI] [PubMed] [Google Scholar]
- 108.Sarda IR, Gorwill RH. Hormonal studies in pregnancy. I. Total unconju-gated estrogens in maternal peripheral vein, cord vein, and cord artery serum at delivery. Am. J. Obstet. Gynecol. 1976;124:234–38. [PubMed] [Google Scholar]
- 109.Schenk F, Brandner C. Indirect effects of peri- and postnatal choline treatment on place-learning abilities in rat. Psychobiology. 1995;23:302–13. [Google Scholar]
- 110.Schwahn BC, Chen Z, Laryea MD, Wendel U, Lussier-Cacan S, et al. Homocysteine-betaine interactions in a murine model of 5,10-methylenetetrahydrofolate reductase deficiency. FASEB J. 2003;17:512–14. doi: 10.1096/fj.02-0456fje. [DOI] [PubMed] [Google Scholar]
- 111.Selhub J, Seyoum E, Pomfret EA, Zeisel SH. Effects of choline deficiency and methotrexate treatment upon liver fo-late content and distribution. Cancer Res. 1991;51:16–21. [PubMed] [Google Scholar]
- 112.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am. J. Epidemiol. 2004;160:102–9. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- 113.Sheard NF, Tayek JA, Bistrian BR, Blackburn GL, Zeisel SH. Plasma choline concentration in humans fed parenterally. Am. J. Clin. Nutr. 1986;43:219–24. doi: 10.1093/ajcn/43.2.219. [DOI] [PubMed] [Google Scholar]
- 114.Shelnutt KP, Kauwell GP, Chapman CM, Gregory JF, 3rd, Maneval DR, et al. Folate status response to controlled fo-late intake is affected by the methylenetetrahydrofolate reductase 677C → T polymorphism in young women. J. Nutr. 2003;133:4107–11. doi: 10.1093/jn/133.12.4107. [DOI] [PubMed] [Google Scholar]
- 115.Shields DJ, Lingrell S, Agellon LB, Brosnan JT, Vance DE. Localization-independent regulation of homocysteine secretion by phosphatidylethanolamine N-methyltransferase. J. Biol. Chem. 2005;280:27339–44. doi: 10.1074/jbc.M504658200. [DOI] [PubMed] [Google Scholar]
- 116.Shin OH, Mar MH, Albright CD, Citarella MT, daCosta KA, Zeisel SH. Methyl-group donors cannot prevent apoptotic death of rat hepatocytes induced by choline-deficiency. J. Cell. Biochem. 1997;64:196–208. doi: 10.1002/(sici)1097-4644(199702)64:2<196::aid-jcb3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 117.Shivapurkar N, Poirier LA. Tissue levels of S-adenosylmethionine and S-adenosylhomocysteine in rats fed methyldeficient, amino acid–defined diets for one to five weeks. Carcinogenesis. 1983;4:1051–57. doi: 10.1093/carcin/4.8.1051. [DOI] [PubMed] [Google Scholar]
- 118.Shronts EP. Essential nature of choline with implications for total parenteral nutrition. J. Am. Diet. Assoc. 1997;97:639–46. doi: 10.1016/S0002-8223(97)00161-2. 49; quiz 47–48. [DOI] [PubMed] [Google Scholar]
- 119.Sitaram N, Weingartner H, Caine ED, Gillin JC. Choline: selective enhancement of serial learning and encoding of low imagery words in man. Life Sci. 1978;22:1555–60. doi: 10.1016/0024-3205(78)90011-5. [DOI] [PubMed] [Google Scholar]
- 120.Song J, da Costa KA, Fischer L, Kohlmeier M, Kwock L, et al. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J. 2005;19:1266–71. doi: 10.1096/fj.04-3580com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Spiers P, Myers D, Hochanadel G, Lieberman H, Wurtman R. Citicoline improves verbal memory in aging. Arch. Neurol. 1996;53:441–48. doi: 10.1001/archneur.1996.00550050071026. [DOI] [PubMed] [Google Scholar]
- 122.Steenge GR, Verhoef P, Katan MB. Betaine supplementation lowers plasma homocysteine in healthy men and women. J. Nutr. 2003;133:1291–95. doi: 10.1093/jn/133.5.1291. [DOI] [PubMed] [Google Scholar]
- 123.Sunden S, Renduchintala M, Park E, Miklasz S, Garrow T. Betainehomocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene. Arch. Biochem. Biophys. 1997;345:171–74. doi: 10.1006/abbi.1997.0246. [DOI] [PubMed] [Google Scholar]
- 124.Sweiry JH, Page KR, Dacke CG, Abramovich DR, Yudilevich DL. Evidence of saturable uptake mechanisms at maternal and fetal sides of the perfused human placenta by rapid paired-tracer dilution: studies with calcium and choline. J. Devel. Physiol. 1986;8:435–45. [PubMed] [Google Scholar]
- 125.Sweiry JH, Yudilevich DL. Characterization of choline transport at maternal and fetal interfaces of the perfused guinea-pig placenta. J. Physiol. 1985;366:251–66. doi: 10.1113/jphysiol.1985.sp015795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tayek JA, Bistrian B, Sheard NF, Zeisel SH, Blackburn GL. Abnormal liver function in malnourished patients receiving total parenteral nutrition: a prospective randomized study. J. Am. Coll. Nutr. 1990;9:76–83. doi: 10.1080/07315724.1990.10720353. [DOI] [PubMed] [Google Scholar]
- 127.Tees RC. The influences of rearing environment and neonatal choline dietary supplementation on spatial learning and memory in adult rats. Behav. Brain Res. 1999;105:173–88. doi: 10.1016/s0166-4328(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 128.Tees RC. The influences of sex, rearing environment, and neonatal choline dietary supplementation on spatial and nonspatial learning and memory in adult rats. Dev. Psychobiol. 1999;35:328–42. doi: 10.1002/(sici)1098-2302(199912)35:4<328::aid-dev7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 129.Tees RC, Mohammadi E, Adam TJ. Altering the impact of early rearing on the rat's spatial memory with pre- and post-natal choline supplementation. Soc. Neurosci. Abstr. 1999;17:1401. [Google Scholar]
- 130.Tessitore L, Sesca E, Greco M, Pani P, Dianzani M. Sexually differentiated response to choline in choline deficiency and ethionine intoxication. Int. J. Exp. Path. 1995;76:125–29. [PMC free article] [PubMed] [Google Scholar]
- 131.Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol. Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 132.Thomas JD, O'Neill TM, Dominguez HD. Perinatal choline supplementation does not mitigate motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicol. Teratol. 2004;26:223–29. doi: 10.1016/j.ntt.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 133.Trommer BA, Schmidt DE, Wecker L. Exogenous choline enhances the synthesis of acetylcholine only under conditions of increased cholinergic neuronal activity. J. Neurochem. 1982;39:1704–9. doi: 10.1111/j.1471-4159.1982.tb08006.x. [DOI] [PubMed] [Google Scholar]
- 134.Tsujiuchi T, Tsutsumi M, Sasaki Y, Takahama M, Konishi Y. Hypomethylation of CpG sites and c-myc gene over-expression in hepatocellular carcinomas, but not hyperplastic nodules, induced by a choline-deficient L-amino acid–defined diet in rats. Jpn. J. Cancer Res. 1999;90:909–13. doi: 10.1111/j.1349-7006.1999.tb00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ulus IH, Wurtman RJ, Mauron C, Blusztajn JK. Choline increases acetylcholine release and protects against the stimulation-induced decrease in phosphatide levels within membranes of rat corpus striatum. Brain Res. 1989;484:217–27. doi: 10.1016/0006-8993(89)90364-8. [DOI] [PubMed] [Google Scholar]
- 136.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse den-tate gyrus. Nat. Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 137.Vance DE. Boehringer Mannheim Award lecture. Phosphatidylcholine metabolism: masochistic enzymology, metabolic regulation, and lipoprotein assembly. Biochem. Cell. Biol. 1990;68:1151–65. doi: 10.1139/o90-172. [DOI] [PubMed] [Google Scholar]
- 138.Vance DE, Walkey CJ, Cui Z. Phosphatidylethanolamine N-methyltransferase from liver. Biochim. Biophys. Acta. 1997;1348:142–50. doi: 10.1016/s0005-2760(97)00108-2. [DOI] [PubMed] [Google Scholar]
- 139.Varela-Moreiras G, Ragel C, Perez de Miguelsanz J. Choline deficiency and methotrexate treatment induces marked but reversible changes in hepatic folate concentrations, serum homocysteine and DNA methylation rates in rats. J. Am. Coll. Nutr. 1995;14:480–85. doi: 10.1080/07315724.1995.10718539. [DOI] [PubMed] [Google Scholar]
- 140.Varela-Moreiras G, Selhub J, da Costa K, Zeisel SH. Effect of chronic choline deficiency in rats on liver folate content and distribution. J. Nutr. Biochem. 1992;3:519–22. [Google Scholar]
- 141.Velzing-Aarts FV, Holm PI, Fokkema MR, van der Dijs FP, Ueland PM, Muskiet FA. Plasma choline and betaine and their relation to plasma homocysteine in normal pregnancy. Am. J. Clin. Nutr. 2005;81:1383–89. doi: 10.1093/ajcn/81.6.1383. [DOI] [PubMed] [Google Scholar]
- 142.Vigo C, Vance DE. Effect of diethylstilboestrol on phosphatidylcholine biosynthesis in the liver of roosters. Biochem. Soc. Trans. 1981;9:98–99. doi: 10.1042/bst0090098. [DOI] [PubMed] [Google Scholar]
- 143.Waite KA, Cabilio NR, Vance DE. Choline deficiency-induced liver damage is reversible in Pemt(−/−) mice. J. Nutr. 2002;132:68–71. doi: 10.1093/jn/132.1.68. [DOI] [PubMed] [Google Scholar]
- 144.Walkey CJ, Yu L, Agellon LB, Vance DE. Biochemical and evolutionary significance of phospholipid methylation. J. Biol. Chem. 1998;273:27043–46. doi: 10.1074/jbc.273.42.27043. [DOI] [PubMed] [Google Scholar]
- 145.Watkins D, Ru M, Hwang HY, Kim CD, Murray A, et al. Hyperhomocysteinemia due to methionine synthase deficiency, cblG: structure of the MTR gene, genotype diversity, and recognition of a common mutation, P1173L. Am. J. Hum. Genet. 2002;71:143–53. doi: 10.1086/341354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wecker L. The synthesis and release of acetylcholine by depolarized hippocampal slices is increased by increased choline available in vitro prior to stimulation. J. Neurochem. 1991;57:1119–27. doi: 10.1111/j.1471-4159.1991.tb08269.x. [DOI] [PubMed] [Google Scholar]
- 147.Weinstein HC, Teunisse S, van Gool WA. Tetrahydroaminoacridine and lecithin in the treatment of Alzheimer's disease. Effect on cognition, functioning in daily life, behavioural disturbances and burden experienced by the carers. J. Neurol. 1991;238:34–38. doi: 10.1007/BF00319708. [DOI] [PubMed] [Google Scholar]
- 148.Weisberg IS, Jacques PF, Selhub J, Bostom AG, Chen Z, et al. The 1298A → C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression and association with homocysteine. Atherosclerosis. 2001;156:409–15. doi: 10.1016/s0021-9150(00)00671-7. [DOI] [PubMed] [Google Scholar]
- 149.Weisgraber KH, Mahley RW. Human apolipoprotein E: the Alzheimer's disease connection. FASEB J. 1996;10:1485–94. doi: 10.1096/fasebj.10.13.8940294. [DOI] [PubMed] [Google Scholar]
- 150.Wendel U, Bremer H. Betaine in the treatment of homocystinuria due to 5,10-methylenetetrahydrofolate reductase deficiency. Eur. J. Pediatr. 1984;142:147–50. doi: 10.1007/BF00445602. [DOI] [PubMed] [Google Scholar]
- 151.Wilcken D, Wang X, Sim A, McCredie R. Distribution in healthy and coronary populations of the methylenetetrahydrofolate reductase (MTHFR) C677T mutation. Arterioscler. Thromb. Vasc. Biol. 1996;16:878–82. doi: 10.1161/01.atv.16.7.878. [DOI] [PubMed] [Google Scholar]
- 152.Williams CL, Meck WH, Heyer DD, Loy R. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res. 1998;794:225–38. doi: 10.1016/s0006-8993(98)00229-7. [DOI] [PubMed] [Google Scholar]
- 153.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12:949–57. [PubMed] [Google Scholar]
- 154.Yang EK, Blusztajn JK, Pomfret EA, Zeisel SH. Rat and human mam-mary tissue can synthesize choline moiety via the methylation of phosphatidylethanolamine. Biochem. J. 1988;256:821–28. doi: 10.1042/bj2560821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem. 1988;263:2998–3004. [PubMed] [Google Scholar]
- 156.Yao ZM, Vance DE. Head group specificity in the requirement of phosphatidylcholine biosynthesis for very low density lipoprotein secretion from cultured hepatocytes. J. Biol. Chem. 1989;264:11373–80. [PubMed] [Google Scholar]
- 157.Young DL. Estradiol- and testosterone-induced alterations in phosphatidylcholine and triglyceride synthesis in hepatic endoplasmic reticulum. J. Lipid Res. 1971;12:590–95. [PubMed] [Google Scholar]
- 158.Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu. Rev. Nutr. 1994;14:269–96. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- 159.Zeisel SH, daCosta K-A, Franklin PD, Alexander EA, Lamont JT, et al. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–98. [PubMed] [Google Scholar]
- 160.Zeisel SH, Mar M-H, Howe JC, Holden JM. Erratum: concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003;133:1302–7. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]; J. Nutr. 133:2918–19. [Google Scholar]
- 161.Zeisel SH, Mar M-H, Zhou Z-W, da Costa K-A. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J. Nutr. 1995;125:3049–54. doi: 10.1093/jn/125.12.3049. [DOI] [PubMed] [Google Scholar]
- 162.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003;133:1302–7. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 163.Zeisel SH, Wurtman RJ. Developmental changes in rat blood choline concentration. Biochem. J. 1981;198:565–70. doi: 10.1042/bj1980565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zeisel SH, Zola T, daCosta K, Pomfret EA. Effect of choline deficiency on S-adenosylmethionine and methionine concentrations in rat liver. Biochem. J. 1989;259:725–29. doi: 10.1042/bj2590725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhu X, Mar MH, Song J, Zeisel SH. Deletion of the Pemt gene increases progenitor cell mitosis, DNA and protein methylation and decreases calretinin expression in embryonic day 17 mouse hippocampus. Brain Res. Dev. Brain Res. 2004;149:121–29. doi: 10.1016/j.devbrainres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 166.Zhu X, Song J, Mar MH, Edwards LJ, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) knockout mice have hepatic steatosis and abnormal hepatic choline metabolite concentrations despite ingesting a recommended dietary intake of choline. Biochem. J. 2003;370:987–93. doi: 10.1042/BJ20021523. [DOI] [PMC free article] [PubMed] [Google Scholar]