Figure 3.
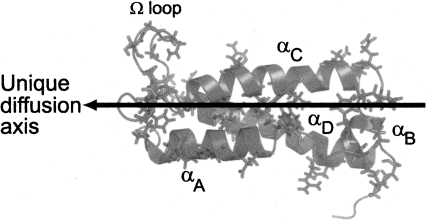
Prolate shape and global folding process of TrAvrPto. TrAvrPto is a prolate molecule, whose unique diffusion axis (shown as an arrow) is aligned with helices αA, αC, and αD. Forty-one slowly exchanging amide groups were identified and are shown mapped onto the structure of TrAvrPto as side chains. They are distributed across all secondary structural elements of TrAvrPto, implying that folding occurs globally: Q35, L37, H41, and E45 of helix αA; A47 and G48 of loop-AB; D52, H54, and E55 of αB; S58, S59, and A61 of loop-BC; Q63, S64, N67, L72, Y73, T76, R78, and L80 of αC; Q86, H87, M90, T91, G92, S94, G95, N97, G99, and L101 of the Ω-loop; H103, E104, N105, M109, R110, A112, W116 ε1, R120, and E121 of αC; and G128 and I129 on the C-terminal tail. Image rendered with PyMOL (DeLano Scientific) using NMR structure (PDB file 1R5E) (Wulf et al. 2004), ensemble-averaged via MOLMOL (Koradi et al. 1996).