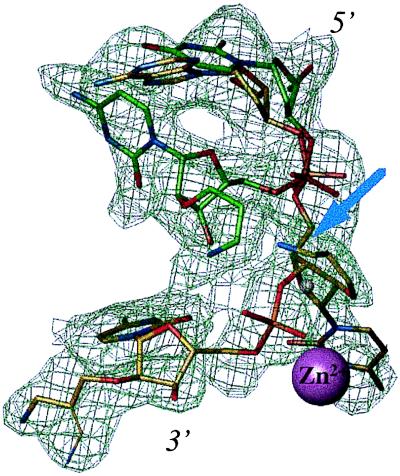
Figure 5.
Alternative orientations of the 5′-d(TCG)-AP(AUC)-3′ hexamer [only AP(AUC) portion visible] at the wt-KF exonuclease active site, surrounded by difference electron density before incorporation of the substrate model (2.2 Å, 1 σ level). Orientation 1 (carbon atoms in yellow) with all three modified 3′ terminal residues present at the active site. The AP substituent of residue A4 (top) was not defined in the electron density map and the substituent of residue C6 adopts two alternative conformations (bottom). The arrow highlights the position of the amino nitrogen of the AP substituent from residue U5 that is located at ca. 1.5 Å from the former position of metal ion B in the wt-KF DNA complex (ref. 30, indicated by a small gray sphere). Orientation 2 (carbon atoms in green) with only two 3′ terminal residues bound at the active site. The AP substituent of residue U5 was not defined in this arrangement. Atoms are colored red, blue, and orange for oxygen, nitrogen, and phosphorus, respectively, and the Zn2+ ion at site A is depicted as a purple sphere.