Figure 5. Brains of DHA-Depleted Tg2576 Mice Show Loss of p85 Expression and Phospho-BAD, Corresponding to Increased Protein Oxidation.
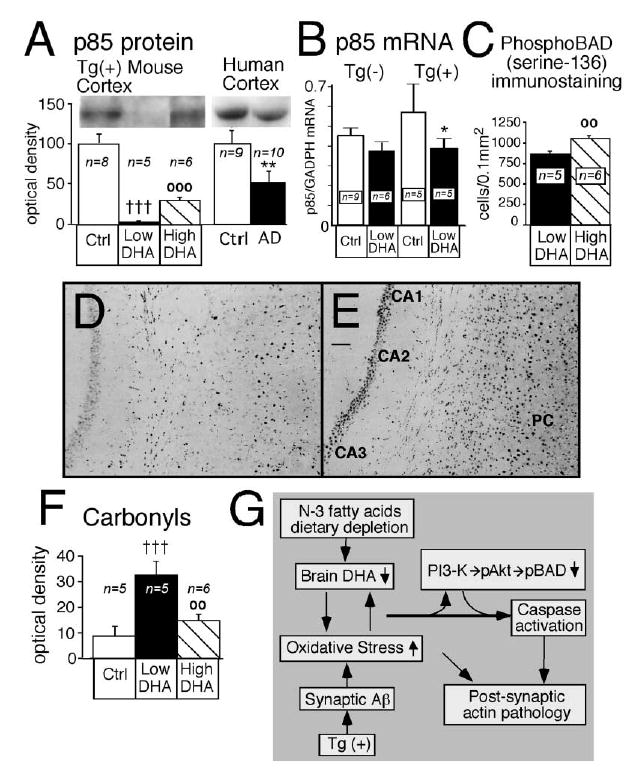
Docosahexaenoic acid (High DHA) increased p85 expression, increased phosphorylation of BAD, and reversed cortical oxidative damage induced by n-3 PFA depletion (Low DHA) in Tg2576 [Tg(+)] mice. (A) The p85α regulatory subunit of phosphatidylinositol 3 (PI3)-kinase in cortex of Tg(+) mice and in temporal cortex samples from human control and AD patients. **p < 0.01 versus human controls, †††p < 0.001 versus Tg(+) Ctrl, and ○○○p < 0.001 versus Tg(+) on n-3 PFA depletion (Low DHA). (B) Quantitative real-time RT-PCR measurements of p85α subunit mRNA in cortex of Tg(−) and Tg(+) mice fed with control diet or low DHA diet. *p < 0.05 versus Tg(+) Ctrl. (C) Image analysis quantification of phospho-BAD in cortex and hippocampus combined. ○○p < 0.01 versus Tg(+) on n-3 PFA depletion (Low DHA). (D and E) Representative examples of phospho-BAD immunostaining in hippocampus (CA1, CA2, and CA3) and parietal cortex (PC) of two mice. The two mice were depleted of N-3 (D) but the mouse in (E) received a diet that was enriched in DHA. Magnification bar, 0.3 mm. (F) The effect of DHA on cortical oxidized proteins levels. †††p < 0.001 versus Tg(+) Ctrl, and ○○p < 0.01 versus Tg(+) on n-3 PFA depletion (Low DHA). (G) Proposed mechanistic pathway for the transgene- and DHA-dependent effect on postsynaptic markers. Aβ overexpression, combined with low n-3 polyunsaturated fatty acid dietary intake, generates an autocatalytic vicious cycle in the postsynaptic dendrites leading to a further increase of oxidative stress and decrease of DHA. This could lead to decreased PI3-kinase activity, caspase activation, further oxidative damage, and consequent breakdown of dendrite spine F-actin filaments and postsynaptic damage.
