Figure 7.
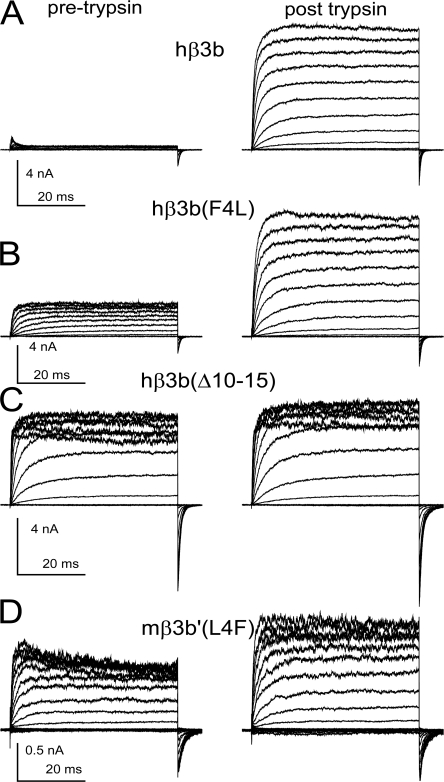
Both residue and length variation contribute to differences in inactivation behavior between mouse β3b' and human β3b subunits. (A) BK channels containing hβ3b subunits were activated by 10 μM Ca2+ with voltage steps applied in 20-mV steps up to +180 mV with tail currents at −120 mV. Currents in the left column were obtained in control saline and, on the right, after brief application of 0.1 mg/ml trypsin. (B) Currents arising from a construct containing a substitution of phenylalanine with leucine (hβ3b-F4L) are shown before and after trypsin. Inactivation is less ineffective but still persists. (C) In construct hβ3b(Δ10-15), residues 10–15 were deleted from hβ3b, resulting in some attenuation of fast inactivation, but also conferring resistant of the residual inactivation to digestion by trypsin. (D) Leucine 4 in mβ3b' was mutated to phenylalanine (mβ3b-L4F), resulting in slight restoration of block at more positive voltages. Consistent with the absence of trypsin sensitivity of the shortened hβ3b(Δ10-15) in C, the block of outward currents in mβ3b'-L4F shows only weak sensitivity to digestion by trypsin.