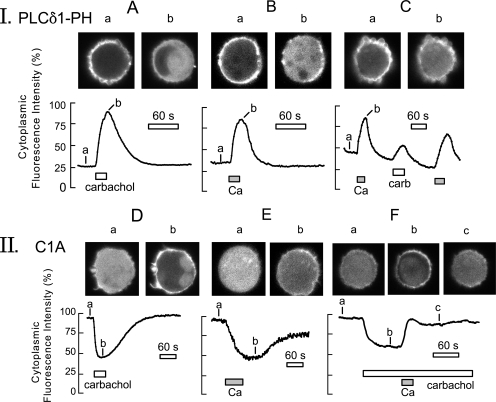
Figure 6.
PLC activation monitored via PLCδ1PH-GFP (panel I) and C1-GFP (panel II) protein fusions in BHK cells voltage clamped to 0 mV via large-diameter pipette tips. The continuous records give the average fluorescence in a central region of the cytoplasm. As evident in micrographs, shown above the cells, the large-diameter tips promote bleb formation. (Panel I, A) The PLCδ1PH-GFP domain, initially localized mostly to the surface membrane, rapidly translocates to the cytoplasm during carbachol (0.3 mM) application for 20 s. When agonist is removed, PH domains return to the cell membrane with a time constant of ∼32 s. (B) Similar PH domain responses recorded when cells are loaded with high Na (40 mM) and Ca influx via NCX1 is activated for 12 s by applying and removing 2 mM extracellular Ca. (C) Comparison of PH domain signals in a cell activated first by Ca influx, then by carbachol, and finally again by Ca. (Panel II, D) The C1 domain is initially cytoplasmic and then rapidly translocates to the membrane during carbachol (0.3 mM) application for 20 s. (E) Slower and smaller C1 domain response, typically obtained during activation of outward NCX1 current. (F) Typical C1 domain cytosolic fluorescence changes when carbachol (0.3 mM) is applied continuously and Ca (2 mM) is then applied for 15 s together with carbachol, followed by washout. C1-GFP domains dissociate rapidly from the plasma membrane during Ca influx and accumulate diffusely in the cytoplasm.