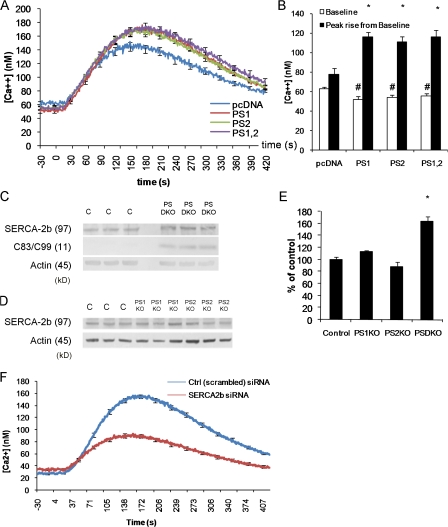
Figure 2.
Ca2+ deficits in presenilin-null fibroblasts are not caused by reduced SERCA2b expression. (A) Changes in cytosolic Ca2+ evoked by thapsigargin in PSDKO fibroblasts transfected 48 h earlier with pcDNA (n = 25 cells), PS1 (n = 40 cells), PS2 (n = 48 cells), or both PS1 and 2 (n = 47 cells), with 0 mM Ca2+ in the bathing solution. Basal cytosolic Ca2+ levels were reduced (∼20 nM) in the presenilin-transfected fibroblasts compared with controls, whereas the peak Ca2+ signals after application of thapsigargin were significantly increased in presenilin-transfected fibroblasts. (B) Mean values of basal cytosolic Ca2+ and thapsigargin-evoked Ca2+ signals, derived from the experiments in A. *, significance in peak rise versus pcDNA (P < 0.05); #, significance in basal levels versus pcDNA (P < 0.05). (C) Steady-state levels of SERCA2b protein are higher in PSDKO fibroblasts compared with controls based on Western blotting. The PSDKO fibroblasts also displayed C83–C99 APP fragments that were not present in the controls because of the lack of γ-secretase activity. β-Actin levels are shown as a loading control. (D) Steady-state levels of SERCA2b in PS1KO and PS2KO fibroblasts compared with controls based on Western blotting. (E) Densitometric analysis of the SERCA2b levels in C and D, normalized to β-actin, showing elevation of SERCA2b levels in the PSDKO fibroblasts. *, significance in peak rise versus pcDNA (P < 0.05; n = 3). (F) Genetic down-regulation of SERCA2b expression lowers ER Ca2+ stores and elevates basal Ca2+ levels. siRNA-mediated down-regulation of SERCA2b leads to lower ER Ca2+ stores (*, P < 0.001; n = 105 cells) when compared with cells treated with a control (scrambled) siRNA. Error bars show SEM.