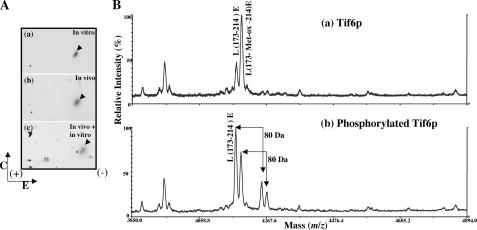
FIGURE 4.
Identification of the phosphopeptide generated from Tif6p labeled with 32P in vivo in yeast cells and in vitro by purified Hrr25p and determination of the phosphorylation site(s). A, HA-tagged Tif6p, labeled with [32P]orthophosphate in yeast cells, was isolated by immunoprecipitation and subjected to SDS-PAGE followed by electrotransfer to a polyvinylidene difluoride membrane. 32P-Labeled Tif6p was located by autoradiography, excised from the membrane, and treated with TPCK-trypsin. The isolated tryptic peptides (100 cpm each) were analyzed by two-dimensional phosphopeptide mapping as described under “Experimental Procedures.” a, 32P-labeled Tif6p phosphorylated in vitro by immunoprecipitated Hrr25p kinase; b, 32P-labeled Tif6p isolated from yeast cells by immunoprecipitation; c, aliquots of 32P-labeled tryptic peptides obtained from a and b were mixed (50 cpm from each panel). The direction of electrophoresis (E) and chromatography (C) are indicated. The sample application origin is indicated by a + sign within the chromatogram. B, MALDI-TOF mass spectra of the Glu-C digested unphosphorylated Tif6p (a) and phosphorylated Tif6p (b). Unphosphorylated and phosphorylated Tif6p were excised from the gel, reduced with 10 mm DTT, alkylated with 55 mm iodoacetamide, and then digested with Glu-C at room temperature overnight. The digestion products were analyzed by MALDI-TOF to identify the phosphopeptides with a PerSeptive MALDI-TOF DE-STR mass spectrometer. In a two ions were observed at 4173.8 and 4189.8 Da corresponding to the mass (m/z) of peptide L173–214E and its oxidized form L173-Met-ox-214E. Two ions corresponding to phosphorylated L173–214E and L173-Met-ox-214E at 4254.2 and 4270.2 Da were observed only from the Glu-C digestion of phosphorylated Tif6p sample in (b).