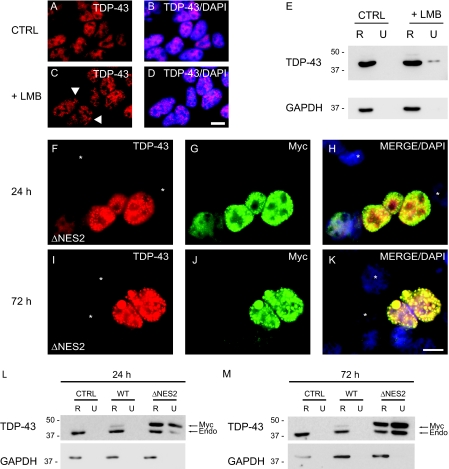
FIGURE 7.
TDP-43 is sequestered as insoluble nuclear inclusions by restricting nuclear export. A-D, immunofluorescence of endogenous TDP-43 (red) alone (A and C) or merged (B and D) with DAPI (blue) in QBI-293 cells treated with DMSO vehicle (CTRL) (A and B) or LMB (+LMB) (C and D). The arrowheads identify punctuate nuclear inclusions in C. E, QBI-293 cells were treated with DMSO vehicle or LMB and were sequentially extracted with RIPA (R) and urea buffer (U). Immunoblotting was conducted with anti-TDP-43 antibody. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. F-K, double labeling with TDP43 (red), Myc (green) antibodies, and DAPI (blue). QBI-293 cells 24 h (F-H) and 72 h (I-K) after transfection with Myc-TDP-43-ΔNES2 (ΔNES2) and merge images (H and K) show colocalization of TDP-43 (red) and Myc (green) in nuclei of transfected cells. Note the presence of punctate inclusions in the nucleus of transfected cells at both 24 and 72 h. Nontransfected cells are marked withanasterisk.LandM, QBI-293 cells 24 h (L) or 72 h (M)post-transfection with empty vector(CTRL), Myc-TDP-43-WT(WT), or Myc-TDP-43-ΔNES2 (ΔNES2) and sequentially extracted with RIPA (R) and urea buffer (U). Immunoblotting was conducted with TDP-43 antibody. Myc-TDP-43 (Myc) migrates slower than endogenous TDP-43 (Endo), and both Myc-TDP-43-ΔNES2 and endogenous TDP-43 were recovered in the RIPA-insoluble and urea-soluble fraction. Glyceraldehyde-3-phosphate dehydrogenase was used as a loading control. Scale bars, 20μm.