FIGURE 3.
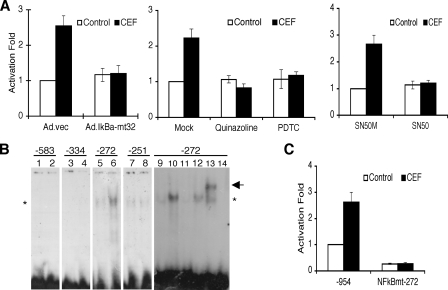
NF-κB is crucial for CEF-induced EAAT2 expression. A, PHFA were infected with Ad.vec or Ad.IκBα-mt32. The infected cells (left) or uninfected PHFA (middle and right) were transfected with EAAT2Pro-954 together with pSV-β-galactosidase as an internal control. One day after transfection, cells were treated with 10 μm CEF together with the indicated inhibitors (middle, 5 μm quinalzoline or 20 μm pyrrolidine dithiocarbamate; right, 5 μm SN50M or SN50) for 2 days. Values are presented as –fold-normalized activity relative to that of the EAAT2Pro-954 in Ad.vec-infected (left), mock-treated (middle), or SN50M-treated (right) cells. B, PHFA were treated with 10 μm CEF for 4 days, and nuclear extracts were prepared. Nuclear extracts were mixed with each radiolabeled oligonucleotide containing the NF-κB binding site located at -583, -334, -272, or -251 of the EAAT2 promoter as follows: lanes 1, 3, 5, 7, and 9, untreated nuclear extracts; lanes 2, 4, 6, 8, and 10–13, CEF-treated nuclear extracts; lane 14, no extracts added. For competition assays, a 100-fold excess of corresponding unlabeled probe (lane 11) or unlabeled probe encompassing the mutated NF-κB site (lane 12) was added. Supershift analysis was performed with an anti-p65 antibody (lane 13). The asterisk and arrow indicate, respectively, NF-κB and a supershift band. C, PHFA were transfected with either the EAAT2Pro-954 or NF-κBmt-272 construct together with pSV-β-galactosidase as an internal control. One day after transfection, cells were treated with 10 μm CEF for 2 days. Values are represented as –fold-normalized activity to that of the EAAT2Pro-954 in untreated cells taken as 1. Error bars indicate S.D.