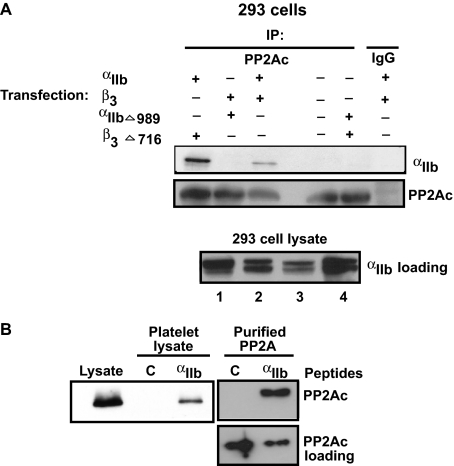
FIGURE 2.
Direct interaction of PP2Ac with αIIb. A, 293 cells were transiently transfected with either wild-type αIIbβ3 or truncated αIIb or β3 mutants as indicated. After 48 h, transfected cells were lysed and immunoprecipitated with control IgG or anti-PP2Ac antibody and immunoblotted with anti-αIIb and anti-PP2Ac antibodies. To demonstrate the presence of αIIb in each of the different transfections performed above, lysates obtained from 293 cells transfected with αIIb- and β3-truncated mutants (1), WT αIIb- and β3-truncated mutants (2), αIIb-truncated mutant and WT β3 (3), and WT αIIbβ3 (4) were immunoblotted with anti-αIIb antibody. B, biotinylated αIIb cytoplasmic peptide (designated “αIIb”) or control (designated C) peptide was incubated with either purified PP2A enzyme or platelet lysates. Proteins were precipitated using streptavidin-agarose beads, separated by SDS-PAGE, and immunoblotted with an anti-PP2Ac antibody. An equal aliquot of peptide/PP2Ac mixture was run simultaneously to demonstrate the presence of PP2Ac among reactions (PP2Ac loading). Blots are representative of three different experiments.