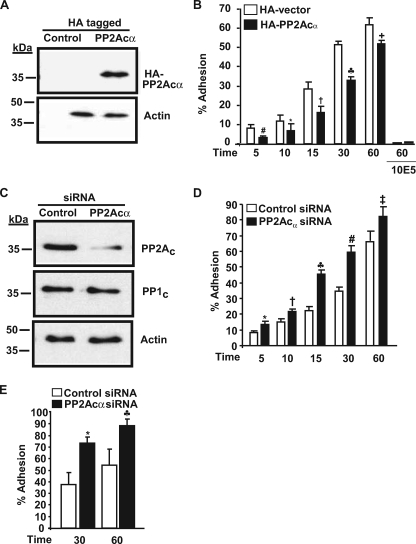
FIGURE 4.
PP2Acα negatively regulates αIIbβ3 adhesiveness in 293 cells. A, PP2Acα expression as revealed by anti-HA antibody in control and HA-tagged PP2Acα-overexpressing cells. This blot was reprobed for actin (loading control) and is a representative of four different experiments. B, effect of HA-PP2Acα overexpression on 293 cell adhesion. 293 cells transfected with control vector or vector with PP2Acα cDNA were allowed to adhere to fibrinogen in the presence and absence of 10E5 (blocking antibody to αIIbβ3), and adhesion was measured by absorbance at 405 nm. Results are mean ± S.E. of 4–7 experiments in triplicate for various time points and 2 experiments for 10E5 blocking studies. Results were significant at #, p = 0.008; *, p = 0.0031; †, p < 0.0001; ♣, p < 0.0001; +, p = 0.002 for 5-, 10-, 15-, 30-, and 60-min adhesion. Error bars were too narrow to be seen with the 10E5 inhibition. C, PP2Ac expression in control and PP2Acα-transfected siRNA. The membrane was reprobed for PP1c and actin to demonstrate siRNA specificity and equal loading respectively. Blots are representative of five different experiments. D, effect of PP2Acα knockdown on 293 cell adhesion to fibrinogen. Results are mean ± S.E. of 5–6 experiments each performed in triplicate and was significant at *, p = 0.001; †, p = 0.006; ♣, p < 0.001; #, p < 0.0001; ‡, p = 0.01 for 5-, 10-, 15-, 30-, and 60-min adhesion by t test. E, effect of PP2Acα knockdown on 293 cell adhesion to von Willebrand factor. Results are mean ± S.E. of three experiments each performed in triplicate and was significant at *, p = 0.0084; ♣, p = 0.0086, for 30 and 60 min.