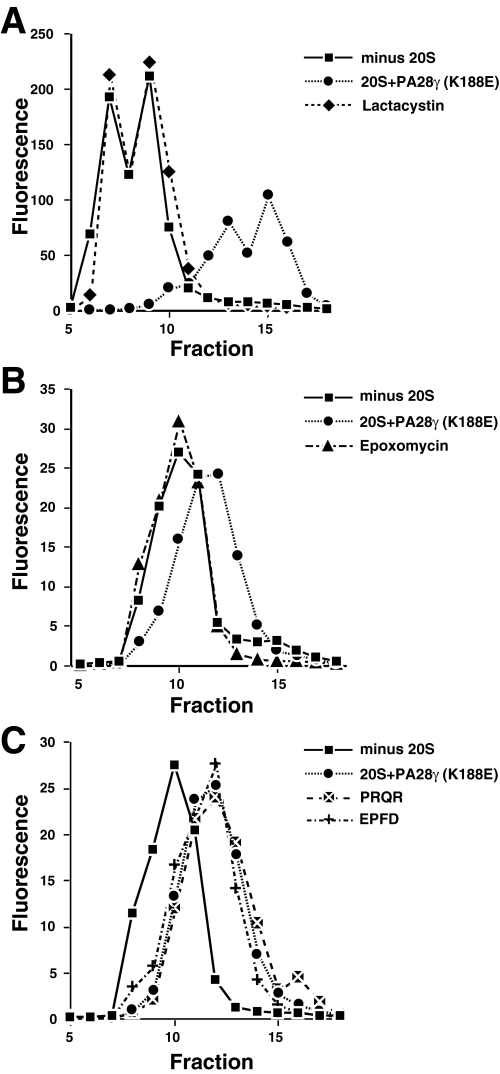
FIGURE 5.
Inhibition of polyQ-peptide degradation by the proteasome inhibitors lactacystin and epoxomicin. Reactions containing 20 S proteasome/PA28γ(K188E) were preincubated with or without proteasome inhibitors for 30 min before adding Fl-polyQ peptide. A, reactions with Fl-HPHQ10RR as substrate were incubated for 6 h before samples were chromatographed on the 10/30 Superdex peptide column. Whereas in the absence of lactacystin Fl-HPHQ7RR was degraded to small fragments (♦), 20 μm of the proteasome inhibitor completely blocked peptide degradation (compare ▪ and •). B, peptide Fl-HPHQ10RR was incubated with 20 S proteasome/PA28γ(K188E) for 3 h in the presence (▴) or absence (♦) of epoxomicin prior to chromatography. Note that epoxomicin completely blocked peptide degradation. C, the peptide Fl-HPHQ7RR was incubated with 20 S proteasome/PA28γ(K188E) in the presence of Ac-PRQR-al (▴) or Ac-EPFD-al (▪) or without any peptide aldehyde (•) for 6 h before chromatography. Note that neither peptide aldehyde inhibited proteolysis of Fl-HPHQ7RR.