Abstract
Disorders of wound healing characterized by impaired or delayed re-epithelialization are a serious medical problem. These conditions affect many tissues, are painful, and are difficult to treat. In this study using cornea as a model, we demonstrate the importance of trefoil factor 3 (TFF3, also known as intestinal trefoil factor) in re-epithelialization of wounds. In two different models of corneal wound healing, alkali- and laser-induced corneal wounding, we analyzed the wound healing process in in vivo as well as in combined in vivo/in vitro model in wild type (Tff3+/+) and Tff3-deficient (Tff3-/-) mice. Furthermore, we topically applied different concentrations of recombinant human TFF3 (rTFF3) peptide on the wounded cornea to determine the efficacy of rTFF3 on corneal wound healing. We found that Tff3 peptide is not expressed in intact corneal epithelium, but its expression is extensively up-regulated after epithelial injury. Re-epithelialization of corneal wounds in Tff3-/- mice is significantly prolonged in comparison to Tff3+/+ mice. In addition, exogenous application of rTFF3 to the alkali-induced corneal wounds accelerates significantly in in vivo and in combined in vivo/in vitro model wound healing in Tff3+/+ and Tff3-/- mice. These findings reveal a pivotal role for Tff3 in corneal wound healing mechanism and have broad implications for developing novel therapeutic strategies for treating nonhealing wounds.
The normal cornea is an avascular transparent tissue and is formed by three cellular layers (epithelial, interstitial/stromal, endothelial cells) which are distinctively separated from each other by two acellular membranes (Bowman and Descemet membrane). The most superficial cell layer is the corneal epithelium. These cells are coated with the tear film on their surface and are separated from the interstitial cell layer (corneal stroma) by Bowman membrane. Descemet membrane is between the interstitial cell layer and the endothelial cell layer.
Deterioration of visual acuity caused by corneal surface diseases such as injuries, keratoconjunctivitis sicca, systemic inflammatory disorders with ocular involvement (e.g. Stevens-Johnson syndrome, ocular cicatricial pemphigoid), and chemical or thermal burns are accompanied by impaired or delayed re-epithelialization of the corneal epithelium. These conditions are difficult to treat and constitute a serious medical problem. Persistent failure of wound healing of the corneal epithelium may lead to corneal ulceration or in more severe cases to perforation, which makes a corneal transplantation inevitable to restore vision (1).
Because of the difficulty of treating corneal blindness once it has occurred, there is a high medical need for early and effective treatment options to enhance corneal wound healing and reduce infection and corneal scarring as a means of decreasing the global burden of corneal blindness. Ocular trauma and corneal ulceration are also significant causes of corneal blindness, accounting for 1.6 million new cases of blindness each year, an additional 2.3 million with bilateral low vision, and 19 million with monocular blindness (2). In addition to accident-, occupation- and sports-related injuries to the eye, elective surgery (radial keratotomy, photorefractive keratectomy, and laser in situ keratomileusis) to correct vision also depend on corneal repair. The physiological and biological process of corneal wound healing has not yet been clarified. However, over the last decade a significant body of research has been performed to unravel the cellular and molecular cascade involved in corneal wound healing. Complex cellular interactions between corneal epithelium, stroma, nerves, immune cells, and lacrimal gland have been described, which are accompanied by expression changes in various kinds of genes such as growth factors (3, 4), cytokines (5, 6), chemokines (7), extracellular matrix molecules (8), and matrix metalloproteinases (9) resulting in proliferation, apoptosis, migration, and differentiation of cells to restore the integrity of the injured cornea.
The mammalian trefoil factor family (TFF)2 comprises three protease-resistant peptides of 7-12 kDa (TFF1 (formerly pS2), TFF2 (formerly hSP), and TFF3 (formerly hP1.B/human intestinal trefoil factor (ITF)) that have a highly conserved distinct motif of six cysteine residues in common, the so-called trefoil domain (10). These peptides can interact with mucins having an influence on mucus viscosity (11), promote migration of epithelial cells in vitro (12, 13), are linked to anti-apoptosis (10, 14), and can induce cell scattering (15, 16), participate in immune response (17, 18), trigger chemotaxis (19) and have further functions (20).
Recent studies have documented the expression of TFF3 in conjunctival globet cells (21) and the epithelium of the nasolacrimal ducts (22). At the mRNA level TFF3 is expressed in healthy cornea but is lacking at the protein level. In contrast, under certain corneal disease states such as keratoconus or herpetic keratitis there is production of TFF3 also at the protein level in the cornea (23).
A putative TFF3 receptor has not yet been identified. However, functional analysis of Tff3 has demonstrated an essential role for Tff3 in the maintenance and repair of the intestinal epithelium (24). In primary rabbit corneal epithelial cells it has been shown in vitro that TFF3 peptide enhances restitution, a multistep process involving the rapid disassembly of cell-cell and cell-substratum contacts, de-differentiation, and spreading of surface cells (25).
Here we demonstrate that Tff3 is induced in corneal epithelial cells after corneal wounding and enhances the re-epithelialization of corneal wounds. Thus, rTFF3 may have a great potential in topical treatment of corneal injury, corneal ulceration, complication of elective corneal surgery, and dry eye syndrome.
EXPERIMENTAL PROCEDURES
Experimental Animals—All animals in this study were treated in accordance with the Association for Research in Vision and Ophthalmology Resolution on the use of Animals in Ophthalmic and Vision Research and the recommendations of the National Institute of Health Guide for the Care and Use of Laboratory Animals. Because the experimental procedures were performed at two different locations in Germany, the experiments were approved by the Ministry of Environment, Nature, and Forests (Schleswig-Holstein, Germany; permit no. V 742-72241.121-1[59-7/03]) as well as by the Department of Country Administration (Sachsen-Anhalt, Germany, permit no. 42502/2-623). The animals were kept under normal controlled laboratory conditions with a 12-h light-dark cycle. BALB/c mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Tff3-/- mice (129Sv background strain) were generated by targeted interruption of the Tff3 gene as described previously (24). Tff3-/- mice and Tff3+/+ mice (129Sv strain, Taconic, Inc., Europe, Denmark) were obtained by interbreeding Tff3+/- mice and carried as separate lineages. Tff3-/- mice are fertile and viable and do not exhibit any overt defects.
Production of Corneal Wounds—To produce corneal wounds, mice were anesthetized by an intramuscular injection of a 1.25% Avertin® (0.2 ml/10g body weight, 2,2,2-tribromoethanol, Sigma-Aldrich). Ophtocain-N® eye drops (Dr. Winzer, Berlin, Germany) were applied to the cornea as a topical anesthetic. 2-mm corneal wounds were produced on the right eye of each animal by (i) placing an alkali-soaked (0.5 m NaOH) filter paper disk (Whatman® filter paper, grade 41, 20-25 μm, Dassel, Germany) of 2 mm diameter on the surface of the cornea for 2 min or (ii) transepithelial excimer laser ablations (2-mm optical zone; 42-44-μm ablation depth, phototherapeutic keratectomy mode) using an Esiris Excimer Laser (Schwind, Aschaffenburg, Germany). The left eye served as a control.
In Vivo and Combined in Vivo/in Vitro Model of Corneal Wound Closure—To evaluate the biological roles of Tff3 in corneal damage and repair and assess the dose and effects of exogenous administered rTFF3, an in vivo and a combined in vivo/in vitro model of corneal epithelial injury in mice were established.
To induce endogenous Tff3 in corneal epithelial cells of BALB/c mice, in vivo 20 BALB/c mice (10 male, 10 female) about 6 month of age and weighting 110-132 g received corneal injury under general anesthesia by placing a 2-min exposure of one eye to a 2-mm filter paper disk soaked in 0.5 m NaOH followed by rinsing in 0.9% sterile saline. The corneas were then allowed to partially heal in vivo for up to 72 h. At the end of each healing period (1, 6, 24, and 72 h; n = 5 at each time point), mice were sacrificed and decapitated, and the heads were fixed in 4% paraformaldehyde for 1 week, decalcified in 10% EDTA, and finally processed for the preparation of paraffin sections.
For the alkali in vivo model in BALB/c mice, corneal injury was induced under general anesthesia by a 2-min exposure of one eye to a 2-mm filter paper disk soaked in 0.5 m NaOH followed by rinsing 0.9% in sterile saline. Ophthalmic drops containing bovine serum albumin (BSA; 0.1 and 1.0 mg/ml) or rTFF3 (0.1, 0.5, 1.0, and 5.0 mg/ml) in phosphate-buffered saline was applied to the wounds twice a day (b.i.d.) for 3 days (n = 5 BALB/c mice per group). Human recombinant glycosylated TFF3 (dimeric molecule) was produced as previously described (12, 26). To assess the healing process, photographs of the corneas were taken at different time intervals post-treatment. On these photographs the corneal defects were evaluated by using a grading system. Each cornea was graded (blinded) for defect intensity (grades 1-5), conjunctival hyperemia (grade 1-5), and light reflection (grades 1-5) and categorized into three groups by adding the grade numbers to an overall score: healed (score 11-15), moderate healing (score 6-10), and defect present (score 3-5). Defect intensity included wound extend and depth. Conjunctival hyperemia was assessed to determine inflammation of surrounding cells. Light reflection depends on opacity of the cornea and smoothness of the corneal surface which, therefore, indicates normal epithelial restitution and regeneration.
For the laser in vivo model in BALB/c mice, corneal ablation was effected with an Excimer laser to the central region of the cornea (2-mm optical zone, 42-44-μm ablation depth) under anesthesia. Ophthalmic drops containing BSA (1.0 mg/ml) or rTFF3 (0.1 and 10 mg/ml) in phosphate-buffered saline was applied to the wounds three times a day (t.i.d.) for 3 days (n = 5 BALB/c mice per group). For the assessment of the wound healing, the above-mentioned grading system was used.
Histology and Immunohistochemistry—For morphological analysis of the cornea of BALB/c, Tff3+/+ and Tff3-/- mice age- and sex-matched mice were decapitated, and the eyes were quickly enucleated, fixed in 4% paraformaldehyde in 0.1 phosphate buffer (pH 7.4), embedded in paraffin, and processed for histology.
Histological and immunohistochemical analysis of the cornea was as previously described (22). For immunolocalization of mouse Tff3, polyclonal anti-rat TFF3 antibody was used (24). This was applied with a standard peroxidase-labeled streptavidin-biotin technique using conventional methods with trypsinization. After counterstaining with hemalum (Merck), the sections were finally mounted in aqueous medium (Aquatex; Roche Applied Science). Two negative control sections were used in each case. One was incubated with the second antibody only; the other with the primary antibody only. Sections of mice small intestine were used for positive control. All slides were examined by microscope (Axiophot, Carl Zeiss, Oberkochen, Germany).
For comparison between Tff3+/+ and Tff3-/- mice (n = 6 Tff3+/+ mice; n = 6 Tff3-/- mice), alkali-induced corneal wounds were induced as described above, and the corneas were allowed to heal in vivo. Healing corneas were photographed at 3, 6, 8, 10, 26, 98, 146, 267, and 462 h. After 462 h, mice were scarified, and ocular bulbi were removed and processed for the preparation of serial paraffin sections that were stained with hematoxylin-eosin and finally analyzed by microscope (Axiophot, Carl Zeiss).
Combination of in Vivo/in Vitro Model of Alkali-induced Corneal Wound in Tff3+/+ and Tff3-/- Mice—After producing alkali-induced corneal wounds (n = 6 Tff3-/- mice; n = 6 Tff3+/+ mice), as described above, the corneas were allowed to partially heal in vivo for 6 or 12 h. For healing in vitro, the ocular bulbi with adhering optic nerves were excised out by surgery and were pinned down on dental wax in 24-well plates using 1-cm-long tips of 20-gauge needles (one eye/well). The eyes were then incubated in serum-free Dulbecco's modified Eagle's medium (0.5 ml/well, PAA Laboratories GmbH, Colbe, Germany) supplemented with 50 μg/ml gentamycin (Invitrogen) in a tissue culture incubator (10% CO2, 90% air, 37 °C) for up to 124 h. After different time points (9, 25, 30, 34, 48, 53, 73, 93, 124 h), post-lesion culture medium was removed, and corneal defects were visualized by dropping fluorescein (Fluoreszein SE Thilo®, Alcon Pharma GmbH, Freiburg, Germany) and photodocumented using an endoscope (Model BA 600; Storz, Tuttlingen, Germany) equipped with a blue light (Storz), a fluorescein filter (Storz), a 0° 2.7-mm rigid optics (Storz) as well as a digital camera (Sony DSC-F717 cyber shot, Tokyo, Japan). After taking photographs of the corneas, fluorescein was removed by rinsing, and new serum-free medium was put into wells.
Treatment with rTFF3 in the Combined in Vivo/in Vitro Model of Alkali-induced Corneal Wound in Tff3+/+ and Tff3-/- Mice—After induction of alkali-induced corneal wounds and partial healing in vivo for 6 h, the ocular bulbi were excised and brought in 24-well plates as described above. The eyes were then incubated in pure serum-free medium (0.5 ml/well) as control or in serum-free medium supplemented with BSA (1.0 mg/ml) as a further control or rTFF3 (0.1, 1.0 & 5.0 mg/ml) in a tissue culture incubator for up to 72 h and allowed to heal in vitro (n = 6 Tff3-/- mice (129Sv background strain) per group and n = 6 Tff3-/- mice (129Sv strain) per group). After 12, 24, 36, 48, 60, and 72 h post-lesion culture medium was removed, and corneal defects were visualized by dropping fluorescein and photodocumented using an endoscope with a digital camera as described above.
In Situ Hybridization—For in situ hybridization for Tff3, mRNA paraffin-embedded eye and colon samples from healthy wild type mice at embryonic days 12.5 of gestation (E12.5), E14.5, E16, E18 and postnatal day 7 (P7) and P14 as well as mice after alkali-induced corneal wounds were used. Fragments of mouse Tff3 cDNA were generated by reverse transcription-PCR from mouse embryo 16.5-day poly(A)+RNA (Sigma) and used to prepare the following subclone in pGEM®-T-Easy-Vektor (Promega): Tff3, base pair 36-238 (Tff3; EMBO data base AQC no. NM_011575). The clone was confirmed by sequencing (MWG Biotech, Ebersberg, Germany). Generation of [35S]UTP-labeled antisense and sense probes from this plasmid by in vitro transcription using T7-RNA polymerase (Sacl) and Sp6-RNA polymerase (Apal) (digoxigenin-labeled kit, Roche Applied Science, #175025910) and in situ hybridization were performed as described by Breitschopf et al. (27).
Scanning Electron Microscopy—To demonstrate the defect created by laser ablation, two corneas were prepared and investigated by scanning electron microscopy, one without defect induction and one after creating a defect by transepithelial excimer laser ablation. For scanning electron microscopy, corneas were impregnated in 2.5% tannic acid for 2 days. A counterfixation in 2% osmium tetroxide for 4 h was followed by dehydration in ethanol and drying in a critical point dryer. Corneas were coated with gold and analyzed with a scanning electron microscope (Philips GmbH, Kassel, Germany).
Statistical Analysis—For the in vivo/in vitro experiments (in vivo/in vitro model of alkali-induced corneal wound in BALB/c, Tff3+/+, and Tff3-/- mice without and with treatment with rTFF3) the corneal defect area was measured for each eye and each time point using the software Sigma Scan Pro 5.0® (Systat, Inc., Richmond, CA).
The primary end point of the study was epithelial wound healing of the cornea in Tff3+/+ versus Tff3-/- mice. The secondary end point was the assessment of rTFF3 concentration required for optimal epithelial wound healing of the injured cornea. After performing Levene and Kolmogorov-Simirnov tests to determine the distribution of the data, we used for the comparison of dependent and independent groups parametric (Student's t test and General Linear Model for repeated measures) and non-parametric (Friedman's test, Mann-Whitney U test) tests, respectively. Post hoc comparisons were performed using the Bonferroni adjustment for multiple comparisons. To avoid a bias of corneal defects at the beginning of the experiments in the different mice groups, we carried out Student's t test and one-way analysis of variance. The wound healing process was estimated by the Kaplan-Meier method, and the difference among groups was verified by the log-rank test. Statistical analysis was performed by means of the statistical software package SPSS 11.5 for Windows®. A p value of <0.05 was defined as statistically significant.
RESULTS
Tff3 Expression in Corneal Epithelium Cells Is Induced after Injury—To determine the expression pattern of Tff3 in injured mice corneas, we exposed the right eye to a 2-mm filter paper disk soaked in 0.5 m NaOH followed by rinsing in sterile saline or treatment of the central cornea with 2 mm excimer laser ablation under general anesthesia. At 1, 6, 24, and 72 h post-injury, 5 mice at each time interval were sacrificed, and the eyes were subjected to immunohistochemical analysis. Tff3 expression was absent in normal BALB/c mouse corneas. Approximately 1 h after corneal injury, Tff3 peptide was detected in the cytoplasm of corneal epithelial cells adjacent to the defect area wounded by alkaline solution (Fig. 1, a-d) as well as by laser (data not shown). In the course of wound healing, the corneal epithelium cells migrating to the injured region showed a more intense immunoreactivity for Tff3 peptide compared with the cells that were 3-5 cells away from the edge of the wound. Even after wound closure, expression of Tff3 was observed up to 72 h in corneal epithelium cells covering the wounded area. The immunostaining pattern of Tff3 during the healing period in corneas with alkali- and laser-induced wound was comparable. Stromal and endothelial cells did not react at any stage with the anti-Tff3 antibody.
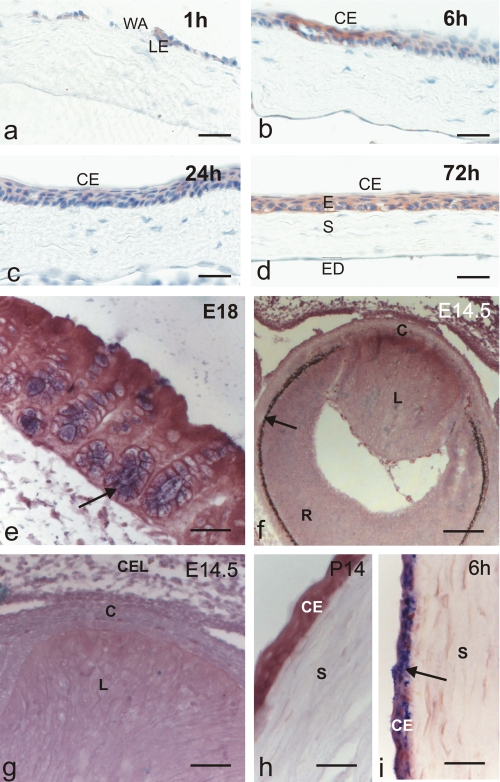
FIGURE 1.
Tff3 protein is expressed in injured corneal epithelium, but Tff3 mRNA is absent during corneal development. Immunohistochemical analysis of Tff3 peptide expression in the course of corneal wound healing after alkali burn (a-d). Wounded corneas were allowed to partially heal in vivo and were then subjected to immunostaining. Tff3 immunoreactivity was detected at the leading edge (LE) of migrating epithelium after 1 h and covering corneal epithelium (CE) up to 72 h after injury. WA, wounded area; E, epithelium, S, stroma; ED, endothelium. In situ hybridization revealed that Tff3 mRNA is expressed during development of wild type mice in goblet cells of colon mucosa (e, arrow, blue) but is lacking during corneal development (f-h). f demonstrates a section through the developing eye at E14.5. The arrow marks retinal pigment epithelium, R, developing retina; L, developing lens; C, developing cornea. g is a magnification of f indicating that no Tff3 transcripts are visible in the developing cornea (C), which is bordered by the developing lens (L) and closed eye lids (CEL) at this stage of development. h shows corneal epithelium (CE) and corneal stroma (S) at P14 lacking Tff3 transcripts. i, Tff3 mRNA is expressed in injured corneal epithelium (arrow, blue)inthe course of corneal wound healing after alkali burn. Wounded corneas were allowed to partially heal in vivo for 6 h and were then subjected to in situ hybridization. Scale bars:48 μmin a-d;32 μmin e; 82.5 μmin f;32 μmin g-i.
Tff3 Transcripts Appear between E16 and E18 in Colon Mucosa but Are Not Expressed during Corneal Development—To determine the question of whether the Tff3 gene is expressed during development and which cells produce Tff3 mRNA upon corneal wounding in situ hybridization on tissues of Tff3+/+ mice was performed. Analysis of the temporal expression pattern revealed that Tff3 transcripts were weakly expressed from E16 onward in goblet cells of colon mucosa, which was used as control and were clearly detectable at E18 (Fig. 1e). This expression pattern could be followed until P14 and was also visible in adult mice. In contrast, no Tff3 mRNA was observed during corneal development at any time point analyzed (Fig. 1, f-h). Healthy adult corneas of Tff3+/+ mice were also devoid of Tff3. However, after corneal injury Tff3 transcripts appeared already very weak approximately after 1 h in corneal epithelial cells of adult mice adjacent to the defect area wounded by alkaline solution. As detectable on protein level (compare Fig. 1, b-d) abundant Tff3 transcripts were also present after wound closure (Fig. 1i) and remained up to 72 h in corneal epithelium cells covering the wounded area. These data are consistent with our previously published results demonstrating TFF3 expression in association with pathological conditions in humans (23). rTFF3 Promotes Corneal Wound Healing in Vivo—To evaluate the biological effects of exogenous administered rTFF3 on corneal wound healing under in vivo conditions, corneas of one eye from 5 BALB/c mice in each treatment group were exposed for 2 min to a 2-mm filter paper disk soaked in 0.5 m NaOH. The contralateral eye was not injured and served as control. Ophthalmic drops, containing BSA as nonspecific protein control at a concentration of 0.1 and 1.0 mg/ml and rTFF3 at different concentrations (0.1, 0.5, 1.0, and 5.0 mg/ml) in phosphate-buffered saline, were topically applied to the corneal wounds twice a day for 3 days. Healing was documented by photographs at several time points during the treatment period (Fig. 2). The photographs taken at 24 h and 72 h after corneal damage were analyzed for the purpose of quantitative and qualitative comparisons between the treatment groups (Fig. 3a). These results demonstrated that topical application of rTFF3 peptide significantly accelerated corneal wound healing in vivo after alkaliburn in a concentration-dependent manner. Fastest re-epithelialization of corneal wounds was obtained at a concentration of 5.0 mg/ml of rTFF3.
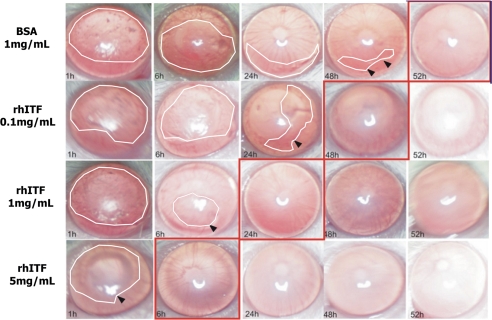
FIGURE 2.
Topical application of rTFF3 enhances corneal wound healing in vivo. Photographs showing alkali-wounded corneas treated with BSA (control) and different concentrations of rTFF3 at five time points during the course of wound healing. Corneas with the earliest evidence of complete wound closure are surrounded by red squares. The edges of the wounds are marked by arrows, and the wound area is surrounded by white lines. These photographs demonstrate a dose-dependent-positive effect of rTFF3 on corneal wound healing in vivo.
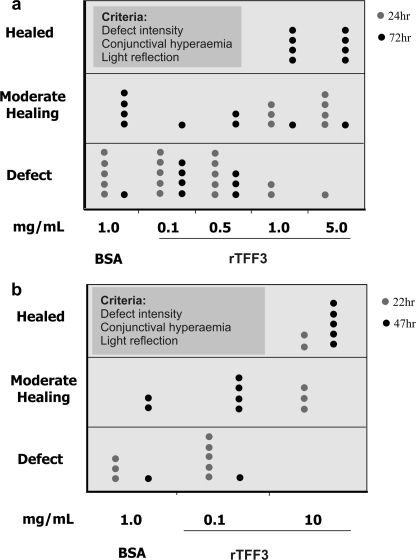
FIGURE 3.
rTFF3 accelerates dose-dependent healing of alkali- and laser-induced corneal defects in vivo. In each treatment group five corneas (one dot represents one cornea) were wounded by alkali solution (a) and laser ablation (b) and allowed to heal in vivo. After 24 h (gray dot) and 72 h (black dot) of treatment with 1.0 mg/ml BSA and 0.1, 0.5, 1.0, and 5.0 mg/ml rTFF3 of the alkali-induced wounds (a) and after 22 h (gray dot) and 47 h (black dot) of treatment with 1.0 BSA and 0.1 and 10 mg/ml rTFF3 of the laser-induced wounds (b), corneas were evaluated for defect intensity (wound depth and extent), conjunctival hyperemia (sign for inflammation), and light reflection (sign for the opacity and smoothness of the cornea) and grouped under three broad categories (see “Experimental Procedures”): Healed, Moderate healing, and Defect. These diagrams demonstrate that the effect of rTFF3 on the date of healing is concentration-dependent.
To study the healing effect of rTFF3 peptide in laser-induced corneal wounds under in vivo conditions, the central region of the cornea from BALB/c mice in each treatment group were injured with an excimer laser. Ophthalmic drops, containing BSA (1.0 mg/ml) or rTFF3 (0.1 and 10.0 mg/ml) in phosphate-buffered saline were applied topically to the corneal wounds 3 times a day for 3 days. The extent of the epithelial defects was documented photographically to monitor the healing progress (data not shown, since images were comparable with the healing process after alkali injury). Qualitative and quantitative analysis showed that rTFF3 at a concentration of 10 mg/ml resulted in optimal healing of laser-induced corneal wounds (Fig. 3b).
Although all laser-induced wounds were verified by direct microscopy, scanning electron microscopy was performed to precisely assess the effect of laser ablation on corneal surface (Fig. 4). The depth of the denuded area immediately after laser application reached in the corneal stroma producing an accurately defined corneal ulcer. Taken together, exogenous administration of rTFF3 peptide on corneal wounds, induced either by alkali or laser, accelerates the re-epithelialization of injured corneal surface.
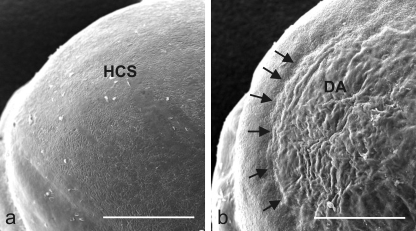
FIGURE 4.
Scanning electron micrographs of mouse corneal surface before (a) and after laser-induced wounding (b). HCS, healthy corneal surface; DA, defect area; arrows mark the defect in the corneal rim. Scale bar, 500 μm.
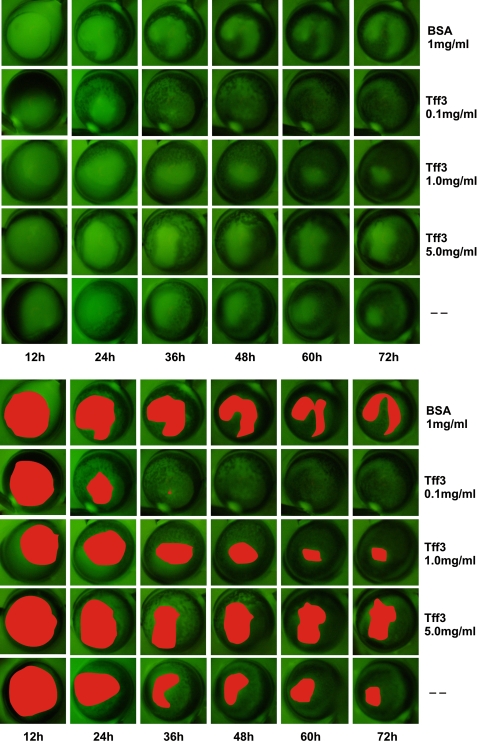
rTFF3 Accelerates Corneal Wound Healing in Combined in Vivo/in Vitro Model—Because Tff3 is produced by conjunctival goblet cells and secreted into the tear film (21), we established a combined in vivo/in vitro model to verify the in vivo data about the therapeutic effects of rTFF3 on re-epithelialization of corneal wounds. In this model alkali-induced corneal wounds were allowed to partially heal in vivo for 6 h followed by enucleation of the eye and cultivation in vitro in serum-free medium containing no additive (control), 1 mg/ml BSA, or rTFF3 (0.1, 1.0, and 5.0 mg/ml) for up to 72 h. Each treatment group consisted of 6 Tff3+/+ mice (129Sv strain). One eye of each mouse was not injured and served as control. Corneal epithelial wounds were visualized by applying a fluorescein solution to the cornea in the course of healing at different time points (Fig. 5). Fluorescein is a orange dye that adheres to corneal stroma and devitalized epithelium cells producing a characteristic green staining under a cobalt-blue filtered light of the wounded area. It normally does not traverse the tight junctions of an intact corneal epithelium. At each time point photographs were taken of the remaining wound areas, and the extent of the epithelial defects was quantified. Statistical analysis showed significant acceleration of corneal wound healing at a dosage of 0.1 mg/ml rTFF3 peptide in comparison to all other concentrations investigated. Collectively, the effects of rTFF3 on corneal wound healing are concentration-dependent and differ in the two models (in vivo versus combined in vivo/in vitro). This point will be addressed under “Discussion.”
FIGURE 5.
rTFF3 promotes re-epithelialization of corneal wounds in combined in vivo (6 h)/in vitro model. Images show representative fluorescein-stained corneal wounds (top panel) treated with BSA (1.0 mg/ml), rTFF3 (0.1, 1.0, and 5.0 mg/ml) and serum-free medium (-, control). After partially healing of the corneal defects in vivo for 6 h, whole eyes were removed from the orbit and transferred into a well plate for the purpose of in vitro cultivation. To quantify the healing progress, the remaining wound areas were digitally photographed at different time points and measured using the software Sigma Scan Pro 5.0®. The measured wound areas are highlighted in the bottom panel.
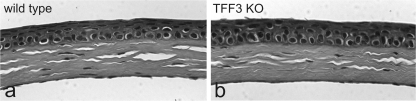
Disruption of Tff3 Gene Does Not Effect Normal Morphological Development of the Cornea—To study the function of Tff3 gene on the development of the cornea, we analyzed adult Tff3-/- and Tff3+/+ mouse corneas after staining with hematoxylin and eosin. Histological examination revealed that the corneas of Tff3-/- mice appeared to have developed normally and were grossly indistinguishable from that in Tff3+/+ animals with respect to morphology, architecture, and thickness (Fig. 6). The corneal epithelium appeared fully stratified with no evidence of growth alterations. There were no noticeable variations in the structure and organization of the Bowman layer, stroma, and endothelium in the Tff3-/- corneas compared with those of the Tff3+/+ animals. These results suggest that in the unwounded state the loss of Tff3 does not appear to disrupt the homeostasis between cell proliferation and cell death required to maintain normal integrity of the cornea.
FIGURE 6.
Morphological development of the cornea is not perturbed in Tff3-/- mice. Histological staining of sections through the cornea of wild type (a) and Tff3 deficient (TFF3 KO (b)) mice. Cell morphology and tissue architecture show no apparent differences.
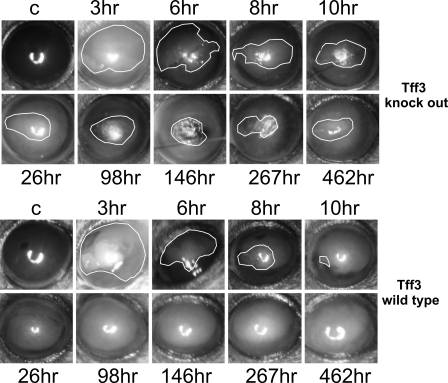
In Tff3-/- Mice, Re-epithelialization of Corneal Defects Is Impaired in in Vivo and in Combined in Vivo/in Vitro Models—To determine whether corneal wound repair is altered in Tff3-/- mice, re-epithelialization after alkali-induced injury to the cornea was quantitatively assess in serial photographs taken at specific intervals during the healing process. First, we compared the wound areas under in vivo conditions in Tff3+/+ and Tff3-/- mice (n = 6 in each group). We found that the wound closure in Tff3-/- mice were impaired (Fig. 7). The recovery from the corneal epithelial injury in Tff3+/+ mice was significantly faster than in Tff3-/- mice. In all Tff3+/+ mice, corneal wound healing was completed by 98 h after injury, whereas in Tff3-/- mice this period was prolonged up to 462 h (p < 0.001). This was partially due to recurrent deterioration of the corneal wounds in 2 of 6 Tff3-/- mice.
FIGURE 7.
Re-epithelialization of corneal wounds in Tff3-/- mice is prolonged in in vivo model. Representative images showing the corneal wound areas (surrounded by white lines) of wild type (upper panel) and Tff3 null mice (bottom panel) taken during the healing period under in vivo conditions at different time points. The first picture in each panel illustrates an unwounded cornea (C, control) of the contralateral eye. In wild type cornea the epithelial wound closure is completed by 10 h after injury, whereas in Tff3 knock out mice wound closure is protracted up to 462 h.
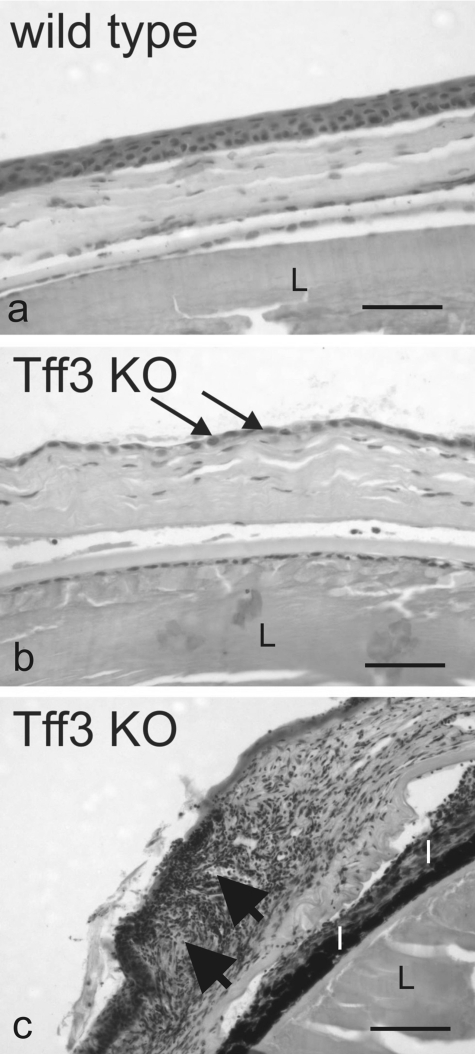
The capacity for repair in Tff3+/+ and Tff3-/- mice was further examined at the histological level after hematoxylin and eosin staining. Although histological examination of uninjured Tff3+/+ and Tff3-/- corneas showed no apparent differences, comparison of alkali wounded corneas on completion of re-epithelialization of the injured area revealed that the multilayer or resurfaced epithelium of Tff3+/+ cornea was reduced to a monolayer of epithelial cells in Tff3-/- mice (Fig. 8). Moreover, in some regions epithelial cells were detached from the underlying stroma, and in other parts the epithelium and stroma of Tff3-/- mice cornea were infiltrated by immune cells, mainly lymphocytes (Fig. 8). This defective corneal wound healing was observed in 3 of 6 Tff3-/- mice after 462 h under in vivo wound healing conditions.
FIGURE 8.
Defective corneal epithelial wound healing in Tff3-deficient mice. Shown are light micrographs taken from the cornea of wild type (a) and Tff3 null (-/-) mice (Tff3 KO, b and c) after macroscopic epithelial wound closure. Histological analysis by hematoxylin and eosin staining of wild type and Tff3-/- mice cornea demonstrates that in Tff3-/- cornea the multilayered organization of the epithelium is thinned to a single layer of epithelial cells (b, arrows) covering the wounded area. Big arrows point to an area of the Tff3-/- cornea infiltrated by immune cells (c). I, iris; L, lens. The scale bar equals to 184 μm.
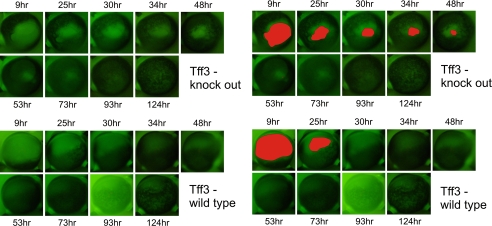
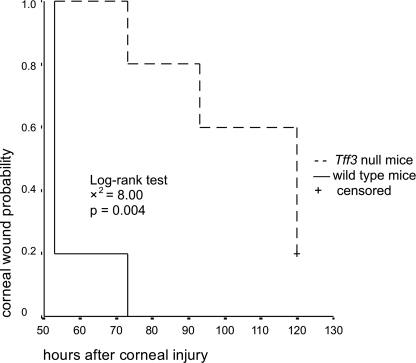
These experiments were also carried out in a combined in vivo/in vitro model. After producing alkali-induced corneal wounds in one eye of Tff3+/+ and Tff3-/- mice (n = 6 in each group), corneas were allowed to partially heal in vivo for 12 h. For healing in vitro, the animals were sacrificed, and the bulbi were enucleated. The eyes were then incubated in serum-free medium for 124 h. During the course of wound healing, the remaining wound areas were visualized by staining with the dye fluorescein (Fig. 9). To compare the corneal wound healing process between the Tff3+/+ and Tff3-/- group, we performed Kaplan-Meier analysis using digitized areas of corneal defects remaining over time. The log-rank test revealed that corneal defects in Tff3-/- mice healed significantly slower than in Tff3+/+ mice (p = 0.004, Fig. 10). This delay in re-epithelialization of corneal wounds in Tff3-/- mice was already evident by 30 h post-injury (p = 0.047).
FIGURE 9.
Re-epithelialization of corneal wounds in Tff3 null mice is prolonged in combined in vivo (12 h)/in vitro model. After alkali-induced corneal wounding and healing in vivo for 12 h, the epithelial defects were stained with fluorescein. Representative images from the cornea of wild type (upper panel) and Tff3 knock out mice (bottom panel) at several time points during wound healing are shown. In the left panels the corresponding remaining wound areas are filled with red color. The epithelial wound in the Tff3 null cornea remains open by 50 h, whereas in wild type cornea the wound is completely closed by 30 h.
FIGURE 10.
Corneal re-epithelialization in Tff3 knock out mice is significantly prolonged. Kaplan-Meier analysis showing probability of corneal wound healing in Tff3+/+ and Tff3-/- mice. Each group consisted of six 7-week-old mice. Corneal epithelial wound healing is significantly accelerated in wild type animals.
rTFF3 Promotes Corneal Wound Healing in Tff3-/- Mice—To determine whether rTFF3 has a beneficial effect on corneal wound healing in Tff3-/- mice (129Sv background strain), 5 Tff3-/- mouse corneas in each treatment group were subjected to epithelial injury by exposing the ocular surface to a 2-mm filter paper disk soaked in 0.5 m NaOH. After a 6-h healing period in vivo, the eyes were enucleated and cultivated in vitro in serum-free medium containing no additive (control) or containing 1 mg/ml BSA or rTFF3 (0.1, 1.0, and 5.0 mg/ml) for an additional 72 h. At several time points the wound areas were photographed and quantified (data not shown). Statistical analysis revealed that an rTFF3 peptide concentration of 0.1 mg/ml had the best effect on corneal re-epithelialization in Tff3-/- mice among all treatment groups.
DISCUSSION
Numerous studies have documented that TFF peptides have many in vivo and in vitro effects on epithelial restitution, wound healing, cell motility, and apoptosis, among others (28). Göke et al. (25) demonstrated that Tff3 enhances the restitution of primary rabbit corneal epithelial cells in vitro. In our previous study we showed that TFF3 is induced in the cornea under certain pathological conditions (23). These results led us to a detailed functional analysis of Tff3 in corneal wound healing. After evaluating the expression pattern of Tff3 during restitution of the corneal surface, we studied the biological effects of rTFF3 on standardized corneal wounds in Tff3 wild type and Tff3 knock-out mice using in vivo and in combined in vivo/in vitro models. Our results demonstrate that the corneal wound healing in Tff3-deficient mice is markedly prolonged compared with wild type mice, and exogenous application of rTFF3 on corneal wounds significantly accelerates the healing process in wild type and Tff3-deficient corneas. In addition, Tff3 probably plays no role during corneal development and in healthy cornea.
The corneal epithelial wound healing is a multistep process that begins with migration of superficial cells to cover the denuded surface, cell proliferation, apoptosis, adhesion, and cell differentiation. This complex cascade is mediated by autocrine and paracrine interactions of cytokines (e.g. interleukin-6, tumor necrosis factor-α) and growth factors (e.g. epidermal growth factor, transforming growth factor-β) produced by the corneal epithelium cells, the stromal cells, the conjunctival cells, and the lacrimal glands. It has been shown that Tff3 is involved in restitution, apoptosis, cell motility, and adhesion (for reviews, see Refs. 29 and 30).
During development and under physiological conditions the corneal epithelium cells do not produce any Tff3. One hour after wounding we detected Tff3 mRNA and protein expression in corneal epithelial cells. This indicates that Tff3 contributes to the very early phase of corneal wound healing. Because TFF3 is synthesized in conjunctivae goblet cells, it is presumed that Tff3 immunoreactivity on corneal epithelial cells may in part arise from secreted Tff3 peptide from goblet cells of the conjunctiva. However, this assumption seems incorrect because only cells adjacent to the wound edge were immunoreactive for Tff3. Another possibility may be that a precursor of Tff3 is stored intracellularly and is activated and secreted after receptor-mediated cell activation.
Local application of rTFF3 peptide promotes the corneal wound healing in in vivo as well as in combined in vitro/in vivo model. We found that a concentration of 5.0 mg/ml rTFF3 peptide under in vivo conditions leads to the most rapid re-epithelialization of the corneal surface, whereas under in vivo/in vitro condition this concentration was only 0.1 mg/ml. This difference in concentration of rTFF3 peptide could be attributed to the fact that eye blinking decreases the concentration of topically applied eye drops. Furthermore, it seems that the loss of Tff3 peptide by eye blinking could not be compensated by the production of Tff3 peptides by conjunctival goblet cells. The result, that under in vivo/in vitro condition a higher concentration of Tff3 peptide does not lead to faster re-epithelialization of the corneal surface, suggests the presence of a receptor for Tff3 peptide. These findings are consistent with the results of Göke et al. (25) that a final concentration of 0.3 mg/ml yielded optimal restitution-enhancing effects of rTFF3 on wounded corneal epithelial cells in vitro.
In Tff3-deficient mice the re-epithelialization of corneal defects is significantly impaired in in vivo model. In two of six corneas the epithelial wounds showed recurrent wound opening. We attributed these occurrences to the formation of only one sheet of epithelium cell layer in Tff3-deficient mice and the fact that these cells were in loose contact to the stroma of the cornea (Fig. 7), leading easily to rewounding with eye blinking. To circumvent this mechanical influence on corneal wound healing in Tff3-deficient mice, alkali-induced corneal wounds were allowed to heal for 6 h in vivo and afterward for up to 124 h in vitro. Under in vivo/in vitro conditions the re-epithelialization of corneal wounds in Tff3-deficient mice were prolonged as compared with wild type mice. Of interest is in this context the finding that TFF3, in contrast to epidermal growth factor, enhances a collective cell migration ensuring a precise coverage of the re-populated wound area avoiding gaps (31).
Collectively, these data suggest that the absence of endogenous Tff3 peptide results in a profound delay of re-epithelialization of corneal wounds. The fact that loss of Tff3 does not disturb the normal development of the cornea supports the concept that Tff3 is required for corneal wound healing but is not essential for the physiological maintenance of the corneal epithelium.
Moreover, the findings have broad implications for developing novel therapeutic strategies in cases after excimer laser surgery (millions of such procedures are performed each year) where a delay in epithelial healing occurs (such a delay is highly undesirable because it puts the cornea at risk of developing postoperative haze, infectious keratitis, and ulceration) and for the treatment of nonhealing wounds such as corneal ulceration, decubitus ulcers, or venous stasis ulcers of the skin (which especially occur in the elderly).
Acknowledgments
We thank Christian Kindler (Helios Klinikum, Erfurt, Germany), Ulrike Bönisch, and Michaela Risch for supporting us in the experimental procedures.
This work was supported, in whole or in part, by National Institutes of Health Grant DK46906 (to D. K. P.). This work was also supported by the GI Co. Inc.; Deutsche Forschungsgemeinschaft Grants PA 738/6-1 and PA 738/9-1 (to F. P.); Bundesministerium für Bildung und Forschung, Wilhelm Roux Program, Halle, Germany, Program Grants FKZ 14/24 and 14/25; and by a Sicca Research Promotion by the Association of German Ophthalmologits (to A. J. and F. P.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TFF, trefoil factor family; TFF3, trefoil factor family peptide 3; rTFF3, recombinant human TFF3; Tff3, mouse trefoil factor family peptide 3; BSA, bovine serum albumin.
References
- 1.Krachmer, J. H., Mannis, M. J., and E. J., H. (1997) in Cornea, (Laibson, P. R., ed) Mosby-Year Book Inc., New York
- 2.Whitcher, J. P., Srinivasan, M., and Upadhyay, M. P. (2001) Bull. W. H. O. 79 214-221 [PMC free article] [PubMed] [Google Scholar]
- 3.Imanishi, J., Kamiyama, K., Iguchi, I., Kita, M., Sotozono, C., and Kinoshita, S. (2000) Prog. Retin. Eye Res. 19 113-129 [DOI] [PubMed] [Google Scholar]
- 4.Klenkler, B., and Sheardown, H. (2004) Exp. Eye Res. 79 677-688 [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita, S., Adachi, W., Sotozono, C., Nishida, K., Yokoi, N., Quantock, A. J., and Okubo, K. (2001) Prog. Retin. Eye Res. 20 639-673 [DOI] [PubMed] [Google Scholar]
- 6.Wilson, S. E., Mohan, R. R., Ambrosio, R., Jr., Hong, J., and Lee, J. (2001) Prog. Retin. Eye Res. 20 625-637 [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto, K., Ikema, K., and Tanihara, H. (2005) Cornea 24 Suppl. 8, S43-S49 [DOI] [PubMed] [Google Scholar]
- 8.Ihanamaki, T., Pelliniemi, L. J., and Vuorio, E. (2004) Prog. Retin. Eye Res. 23 403-434 [DOI] [PubMed] [Google Scholar]
- 9.Kuo, I. C. (2004) Curr. Opin. Ophthalmol. 15 311-315 [DOI] [PubMed] [Google Scholar]
- 10.Taupin, D. R., Kinoshita, K., and Podolsky, D. K. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 799-804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thim, L., Madsen, F., and Poulsen, S. S. (2002) Eur. J. Clin. Investig. 32 519-527 [DOI] [PubMed] [Google Scholar]
- 12.Dignass, A., Lynch-Devaney, K., Kindon, H., Thim, L., and Podolsky, D. K. (1994) J. Clin. Investig. 94 376-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oertel, M., Graness, A., Thim, L., Buhling, F., Kalbacher, H., and Hoffmann, W. (2001) Am. J. Respir. Cell Mol. Biol. 25 418-424 [DOI] [PubMed] [Google Scholar]
- 14.Lalani, E. N., Williams, R., Jayaram, Y., Gilbert, C., Chaudhary, K. S., Siu, L. S., Koumarianou, A., Playford, R., and Stamp, G. W. (1999) Lab. Investig. 79 537-546 [PubMed] [Google Scholar]
- 15.Emami, S., Le Floch, N., Bruyneel, E., Thim, L., May, F., Westley, B., Rio, M., Mareel, M., and Gespach, C. (2001) FASEB J. 15 351-361 [DOI] [PubMed] [Google Scholar]
- 16.Williams, G. R., and Wright, N. A. (1997) Virchows Arch. 431 299-304 [DOI] [PubMed] [Google Scholar]
- 17.Baus-Loncar, M., Kayademir, T., Takaishi, S., and Wang, T. (2005) Cell. Mol. Life Sci. 62 2947-2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook, G. A., Familari, M., Thim, L., and Giraud, A. S. (1999) FEBS Lett. 456 155-159 [DOI] [PubMed] [Google Scholar]
- 19.Chwieralski, C. E., Schnurra, I., Thim, L., and Hoffmann, W. (2004) Am. J. Respir. Cell Mol. Biol. 31 528-537 [DOI] [PubMed] [Google Scholar]
- 20.Blin, N. (2005) Cell. Mol. Life Sci. 62 2907-2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langer, G., Jagla, W., Behrens-Baumann, W., Walter, S., and Hoffmann, W. (1999) Investig. Ophthalmol. Vis. Sci. 40 2220-2224 [PubMed] [Google Scholar]
- 22.Paulsen, F. P., Hinz, M., Schaudig, U., Thale, A. B., and Hoffmann, W. (2002) Investig. Ophthalmol. Vis. Sci. 43 3359-3364 [PubMed] [Google Scholar]
- 23.Steven, P., Schafer, G., Nolle, B., Hinz, M., Hoffmann, W., and Paulsen, F. (2004) Peptides 25 819-825 [DOI] [PubMed] [Google Scholar]
- 24.Mashimo, H., Wu, D. C., Podolsky, D. K., and Fishman, M. C. (1996) Science 274 262-265 [DOI] [PubMed] [Google Scholar]
- 25.Göke, M. N., Cook, J. R., Kunert, K. S., Fini, M. E., Gipson, I. K., and Podolsky, D. K. (2001) Exp. Cell Res. 264 337-344 [DOI] [PubMed] [Google Scholar]
- 26.Babyatsky, M. W., deBeaumont, M., Thim, L., and Podolsky, D. K. (1996) Gastroenterology 110 489-497 [DOI] [PubMed] [Google Scholar]
- 27.Breitschopf, H., Suchanek, B., Gould, R. M., Colman, D. R., and Lassmann, H. (1992) Acta Neuropathol. 84 581-587 [DOI] [PubMed] [Google Scholar]
- 28.Otto, W. R., and Thim, L. (2005) Cell. Mol. Life Sci. 62 2939-2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann, W. (2005) Cell. Mol. Life Sci. 62 2932-2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulsen, F. P., and Berry, M. (2006) Prog. Histochem. Cytochem. 41 1-56 [DOI] [PubMed] [Google Scholar]
- 31.Dürer, U., Hartig, R., Bang, S., Thim, L., and Hoffmann, W. (2007) Cell. Physiol. Biochem. 20 329-346 [DOI] [PubMed] [Google Scholar]