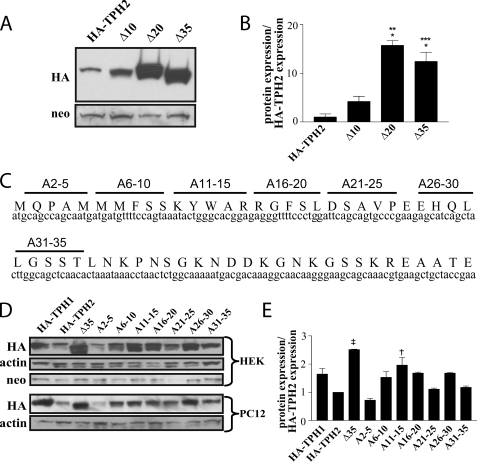
FIGURE 4.
Isolation of the residues containing the regulatory determinant. A and B, Western blots from HEK293 cells expressing HA-TPH2 constructs containing truncations of 10, 20, and 35 residues reveal the bulk of the sequence required for the decreased protein expression lies within the sequence coding for amino acids 10–20. B, results presented in the graph were normalized to neomycin phosphotransferase expression and presented as a ratio to full-length HA-TPH2 expression (n = 3). *, p < 0.001 compared with HA-TPH2; **, p < 0.001 compared with Δ10; ***, p < 0.01 compared with Δ10. C, a schematic illustrating the residues mutated in an alanine-scanning mutagenesis. D, Western blots from both HEK293 and PC12 cells showing expression levels of HA-TPH2 proteins with the indicated residues mutated to alanine support the importance of the sequence coding for residues 10–20 in mediating the decreased protein expression. E, the accompanying graph represents average protein levels in HEK293 cells normalized to neomycin expression and compared with wild type HA-TPH2 expression (n = 3). †, p < 0.001 compared with HA-TPH2; ‡, p < 0.01 compared with HA-TPH2. Data are means ± S.E.