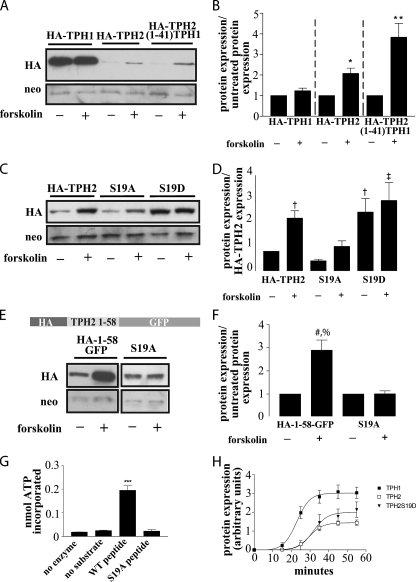
FIGURE 5.
Phosphorylation of Ser19 modulates TPH2 expression. A, a Western blot from HEK293 cells expressing HA-TPH1, HA-TPH2, or the HA-TPH2-(1–41)-TPH1 chimera with and without treatment with 2.5 μm forskolin for 4 h demonstrates a PKA-mediated increase in TPH2 and TPH2-(1–41)-TPH1 expression with no appreciable effect on TPH1 expression. B, bar graph showing the result of n = 5 experiments. *, p < 0.05 compared with untreated control; **, p < 0.001 compared with untreated control. The data in graph B are normalized to neomycin expression and compared with their respective untreated controls. C, a Western blot from HEK293 cells expressing HA-TPH2(S19A) to remove the putative PKA phosphorylation site or S19D to create a pseudophosphorylated protein supports the hypothesis that phosphorylation of Ser19 leads to an increase in protein expression. The responses to forskolin treatment (described above) support this, since the pseudophosphorylated S19D construct is not appreciably affected by forskolin-mediated PKA activation, and the S19A response to forskolin is blunted compared with wild type TPH2. The graph shown in D represents n = 3 experiments. †, p < 0.05 compared with HA-TPH2; ‡, p < 0.01 compared with HA-TPH2. The data in graph D are normalized to neomycin expression and compared with untreated HA-TPH2 expression. E and F, to remove the possible confound of additional PKA phosphorylation sites, the S19A mutation was generated in the HA-(1–58)-GFP construct. Although the expression of the HA-(1–58)-GFP construct increased in response to the previously outlined forskolin treatment, expression of the S19A mutant remained unchanged. The results shown in F represent n = 3 experiments with treated samples normalized to neomycin and compared with the untreated partner sample. #, p < 0.01 compared with HA-(1–58)-GFP; %, p < 0.01 compared with S19A + forskolin. G, phosphorylation of a peptide corresponding to amino acids 10–20 and that same peptide with the S19A mutation confirm the status of Ser19 as a PKA phosphorylation site (n = 3). ***, p < 0.01 compared with “no enzyme,” “no substrate,” and “S19A peptide” controls. H, in vitro translation performed on cDNA in a modified pcDNA vector shows that the S19D mutation does not dramatically alter TPH2 translational efficiency (n = 4). All results are means ± S.E.