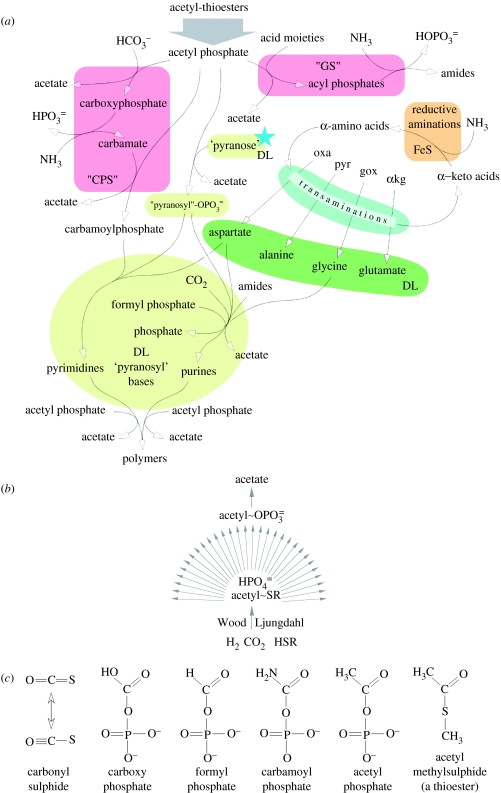
Figure 6.
Speculations about early biochemistry. (a) Possible routes of nitrogen incorporation into metabolism, energetically feasible with acetyl phosphate owing to its higher energy of hydrolysis (table 2) than ATP, which is the modern phosphoryl donor in the enzymes today. Oxalacetate (oxa), pyruvate (pyr), glyoxylate (gox) and α-ketoglutarate (αkg) also occur on the left-hand side of figure 3. ‘Pyranose’ (Eschenmoser 2004) indicates that we do not specify the ancestral kind of sugar phosphate in the backbone of RNA-like polymers. The blue star indicates that we have not specified a mechanism of sugar phosphate formation here, although we suspect it to have proceeded via PEP and PGA, via the enolase reaction (figure 3). ‘DL’ indicates that pyranosyl (or other sugar) phosphate synthesis probably occurred without stereospecificity at first, and that a chemical hypercyclic feedback loop into a handed precursor of the enolase reaction as sketched in figure 7 tipped the homochirality scale for sugars. (b) A reminder that we are suggesting the synthesis of thioesters that are the ‘food’ for an autocatalytic chemical cycle (Hordijk & Steel 2004) to have been continuous and stable and to have proceeded initially with inorganic catalysts only. R indicates a simple aliphatic residue. (c) Structures of some of the simple reactive compounds that are central to the considerations in this paper. The Lewis structures for COS are from Luther (2004).