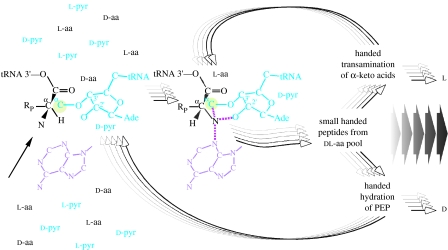
Figure 7.
Suggestion for the origin of homochirality (see text) via an autocatalytic cycle in the sense of Hordijk & Steel (2004). From a mixture of l and d amino acids, only those of one α-carbon configuration are incorporated into protein by virtue of the chance stereochemistry of the initial peptidyl transferase reaction catalysed by a protoribosome. Handed peptides (also possible to synthesize initially by chance, owing to the complexity of racemates with many chiral centres, see text) with some enolase activity produce more d sugars, possibly as pyranose (pyr), leading to more stereoselective peptide synthesis at the peptidyl transferase site of the ribosome as redrawn from Steitz (2005), because only activated amino acids of one configuration will polymerize. This is schematically indicated by the fit of an activated l-amino acid into the modern peptidyl transferase site (centre), where the α-amino group of the next amino acid to be polymerized is coordinated by the active site so as to attack the C-terminal carbonyl carbon of the tRNA-bound growing peptide chain, whereas the d-configuration (left) leaves the amino group in the wrong spot (arrow) for peptidyl transfer. While the translation process can filter one configuration into peptides, it cannot synthesize the l-configuration. But handed peptides with PLP-dependent transaminase activities can (arrows leading to l), feeding back into the autocatalytic cycle by promoting more of both the stereochemically homogeneous enolase and the transaminase activity. Note that the autocatalytic cycle requires a sustained source of new precursors (‘food’) in order to operate (Hordijk & Steel 2004).