Abstract
Study Objective:
To determine if recently abstinent, heavy marijuana (MJ) users show differences in polysomnographic (PSG) measures compared with a drug-free control group.
Design:
A group of carefully selected heavy MJ users were chosen for study inclusion and matched to a drug-free control group. Questionnaire data were collected prior to cessation of MJ use. PSG studies were conducted during 2 consecutive nights after discontinuation of MJ use in our core sleep laboratory.
Setting:
Baltimore Maryland, General Clinical Research Center (GCRC) core sleep lab.
Participants:
17 heavy MJ users discontinuing MJ use and 14 drug-free controls. Men and women were studied, 18 to 30 years. The MJ users reported no other drug use and alcohol use was negligible in both groups. Urine was positive for metabolites of cannabis only.
Measurements and Results:
The MJ users showed differences in PSG measures (lower total sleep times, and less slow wave sleep than the control group) on both nights; they also showed worse sleep efficiency, longer sleep onset, and shorter REM latency than the control group on Night 2. More sleep continuity parameters were significantly worse for the MJ group than the control group on Night 2 versus Night 1, indicating that sleep in the MJ group was relatively worse on Night 2 compared to Night 1. The MJ group did not show improved sleep after an adaptation night as expected. Withdrawal symptoms, craving, and depression did not appear to influence these findings.
Conclusions:
During discontinuation of heavy MJ use, PSG measures of sleep disturbance were detected in MJ users compared with a drug-free control group. While this preliminary study cannot identify the extent to which these group differences were present before abstinence, poor sleep quality either prior to or after MJ discontinuation could result in treatment failure for MJ users. Further investigation is necessary to determine the association between the use and cessation of MJ and sleep disturbance.
Citation:
Bolla KI; Lesage SR; Gamaldo CR; Neubauer DN; Funderburk FR; Cadet JL; David PM; Verdejo-Garcia A; Benbrook AR. Sleep disturbance in heavy marijuana users. SLEEP 2008;31(6):901-908.
Keywords: Marijuana, sleep, polysomnography, withdrawal
PEOPLE WITH SUBSTANCE-RELATED DISORDERS OFTEN EXPERIENCE SLEEP PROBLEMS THAT PERSIST FOR MONTHS AFTER CESSATION OF DRUG USE.1,2 These sleep disturbances could precipitate relapse in recently abstinent substance users as they attempt to improve their sleep quality. Eleven million Americans use marijuana (MJ) either alone, or in conjunction with other illicit drugs, and this number is increasing. Increases in the numbers of MJ users, coupled with increases in potency over recent years have resulted in a higher prevalence of MJ use disorders.3 Except for alcohol, MJ use accounted for the largest percent of drug abuse treatment admissions (15.9%) in 2004. A major problem in the treatment of MJ users is that up to 76% of those who abruptly stop using MJ report disturbed sleep (strange dreams, insomnia, poor sleep quality), possibly increasing the risk of relapse.4
Aside from self-reports of sleep disturbance by recently abstinent MJ users, there are only a handful of studies that have recorded polysomnography (PSG) in MJ users in the past 20 years.5–8 After oral administration of a high dose of MJ extract, REM sleep decreased and slow wave sleep (SWS) increased.5,6 Following one day of no MJ use, REM sleep increased and SWS decreased. In another study, 3 MJ-dependent men (mean age 40 yr) were studied during 3 days of abstinence.7 Over the 3 days, sleep efficiency (total sleep time [TST]/time in bed [TIB] and initial REM latency decreased, while percent REM of TST, SWS (% TST), ratings of MJ craving, and irritability increased. These 2 studies showed contradictory results with respect to SWS, which could be related to differences in the demographic characteristics of the MJ users (e.g., amount of MJ use) or the timing of the PSG in relationship to the number of days of abstinence. Although the numbers of studies are few, these results show robust sleep abnormalities after MJ discontinuation and underscore the need to further investigate sleep disturbance in recently abstinent MJ users. Sleep disturbance in MJ users has important basic science and clinical implications. Furthering our understanding of how sleep is affected in MJ users could provide insights not only into the process of addiction but also into the functioning of the endogenous cannabinoid system, since this system plays a role in sleep promotion.9 In addition, a better understanding of sleep disturbance in recently abstinent MJ users has potential implications for understanding relapse and guiding treatment interventions.
The aim of this study was to determine if MJ users self-reporting sleep disturbance when discontinuing MJ use in the past show objective PSG findings that are different from a drug-free control group. Based on previous subjective reports of sleep disturbance and limited objective PSG findings, we hypothesized that abstinent MJ users would show longer time to sleep onset and more difficulty with sleep maintenance than a drug-free control group. Since we have repeatedly found dose-related associations between the amount and duration of MJ drug use and measures of brain function,10,11 we explored also whether there was an association between the amount and duration of MJ use and the severity of sleep disturbance.
METHODS
Participants
We recruited participants through newspaper advertisements. To control for any medical, neurological or psychiatric conditions, participants received full medical and psychiatric screening. Screening consisted of drug use and psychological history using the Drug Use Survey Questionnaire (DUSQ),12 Addiction Severity Index (ASI),13 and the Psychiatric Diagnostic Interview Schedule (DIS-IV)14 corresponding to the Diagnostic and Statistical Manual of Mental Disorders, Version IV (DSM-IV). Medical screening consisted of complete physical and neurological examinations, including urine toxicology. All participants spoke English as their native language, and all had estimated IQs >85 as assessed by the Shipley Institute of Living Scale.15 The age range for inclusion was greater than 18 years and younger than 30 years. From the DIS-IV it was determined that none of the participants had comorbid Axis I psychiatric disorders or antisocial personality disorder. The Institutional Review Boards of the National Institute on Drug Abuse-Intramural Research Program (NIDA-IRP), the Johns Hopkins Medical Institutions, Joint Committee on Clinical Investigation, and the Johns Hopkins Bayview Medical Institutional Review Board approved this study. All participants provided informed consent and received remuneration.
Marijuana (MJ) Group
This group was comprised of chronic MJ users who claimed MJ as their drug of choice. Inclusion criteria for MJ participants were: reported use of MJ for ≥2 years and smoked MJ '5 times per week over the past 3 months; reported alcohol consumption ≤15 drinks per week; and urine toxicology screen positive for cannabis metabolites and negative for amphetamine, barbiturate, cocaine, methadone, opiate, PCP, and benzodiazepine at the time of screening and admission to the study. Participants reported no regular use of any other illicit drugs. Since our study aim was to determine if we could detect objective PSG findings of subjective reports of sleep disturbance with cessation of MJ use, we purposely biased our selection of MJ users towards individuals who reported disrupted sleep during prior periods of attempted abstinence. Therefore, for inclusion, MJ users also had to report ≥2 withdrawal symptoms on a MJ withdrawal questionnaire with ≥1 being a sleep disturbance symptom upon discontinuation of MJ use in the past. Twenty-nine percent (5/17) of the MJ participants met the DSM-IV diagnosis for cannabis dependence; one MJ user met the diagnosis for cannabis abuse only; and 65% (11/17) of MJ users did not meet the diagnosis for cannabis dependence or abuse. The most common reason that diagnostic criteria were not met was because the MJ users did not report substance-related legal problems or social or interpersonal problems related to drug use. We excluded participants if they met DSM-IV criteria for current or past dependence on any other psychoactive substance, including alcohol. Individuals were not excluded for nicotine dependence, although no participant met criteria for this diagnosis.
Control Group
Control participants qualified if they did not meet DSM-IV criteria for past or current dependence or abuse of any substance except nicotine and tobacco. Reported alcohol consumption was ≤15 drinks per week. A urine toxicology screen prior to testing revealed no use of any illicit drug for all participants in the control group. No participant in the control group reported using MJ in the past 3 months, and lifetime use of MJ was negligible.
Exclusion Criteria
A screening sleep history questionnaire and a personal sleep history interview were conducted by a sleep medicine physician to look for underlying sleep disorders which would exclude a participant from the study. No participant endorsed symptoms of narcolepsy, restless legs syndrome, or periodic leg movements. Participants were excluded if they had medical conditions that may affect sleep architecture, a prior history of sleep disorder, or any neuropsychiatric condition including the following: seizure disorder, dementia, CNS infection, demyelinating disease, space-occupying lesion, movement disorder, CNS vasculitis, autoimmune disease (HIV), head injury with loss of consciousness >5 min, or congenital CNS abnormality. We excluded volunteers if they had a history of hypertension, diabetes, or current use of medications that may affect sleep function (anxiolytics, antidepressants, stimulants, antihistamines, antipsychotics). Since obese individuals are more prone to obstructive sleep apnea, body mass index (BMI) was limited to less than 32 for men and 35 for women. Finally, we excluded those participants who met clinical criteria for sleep apnea (>10 disordered breathing events/h) on the PSG on either Night 1 or Night 2 from the final sample. Subjects with AHI >10/h were excluded to eliminate cases with potentially clinically significant sleep disruption due to sleep disordered breathing. An apnea-hypopnea index (AHI) was selected based on the current literature evaluating presence of SDB in relation to established comorbid risk factors such as stroke, heart disease, congestive heart failure, and arrhythmias. The minimum AHI threshold used in these studies to define SDB was >10/h.16
Data Collection
Prior to the admission date, the study coordinator met with participants and obtained informed consent; she gave the participants sleep diaries and instructed them on their use during the 5 mornings prior to admission. During this initial visit the participants completed the Horne-Ostberg morningness-eveningness scale17 to define individual chronotypes (sleep-wake circadian rhythm pattern) to ensure that the groups did not differ substantially on their sleep-wake trait characteristics and sleep habits. The participants also completed a detailed sleep history questionnaire (SHQ) that was developed at the Johns Hopkins Sleep Center. Section 1 of the SHQ is composed of 6 questions that ask the participants to rate their sleep quality and degree of satisfaction with their sleep/alertness from “very good” to “very poor” (Sleep Satisfaction Score). We assigned values of zero for “very good” to 7 for “very poor.” The next 84 questions (Section 2, Symptoms Related to Disturbed Sleep) characterize the sleep disturbance experienced by the individual during the past few months. Specifically it asks: “how often do you find that you ….doze or nod off at work,” “have restless sleep,” or “use marijuana to help you sleep.” The 5 response choices range from “never” to “almost always.” We assigned a numerical score to each response with “never” receiving a score of zero and “almost always” receiving a score of 5. We summed the items for each section. The higher the score, the lower the sleep satisfaction (Section 1) and the more frequent the symptoms related to disturbed sleep (Section 2). These measures were collected prior to MJ discontinuation and admission into a controlled environment. We instructed the MJ participants not to deviate from their normal sleep-wake and MJ smoking routines (all smoked MJ daily) until the time of their admission. When questioned about their last MJ use upon admission, 7 MJ users smoked the morning of admission, 7 smoked the day before, and 3 smoked within 48 h of admission.
On the day of admission into the GCRC, a sleep medicine physician interviewed each participant to screen for any past history or baseline sleep problems and performed a physical. After admission, the MJ participants resided in the Clinical Inpatient Research Unit (CIRU) at NIDA-IRP where abstinence was enforced during 14 days. Control participants resided in the GCRC sleep laboratory for 3 days. During this time, standard PSG recordings were performed on Nights 1, 2, 7, 8, and 13 for MJ users, and Nights 1 and 2 for control participants. Time for lights out was determined by taking the median time from the previous 5 nights of sleep after review of the sleep logs with the participants. Subjects were then given the opportunity to sleep 8 h from the time of lights out. This method was used to avoid putting subjects to bed at a time uncharacteristic of their normal sleep habits (because the study was designed to test sleep problems that were different from an individual's norms instead of societal normative standards). After admission to the CIRU, all participants having sleep studies did not receive any food or beverage containing caffeine. In addition, cigarette smoking was allowed only before 19:00, and only when escorted by a staff member to an outside designated smoking area. Only 2 nights of PSG recordings are presented in this report because the control group only had PSG studies on Nights 1 and 2, and thus group differences could only be determined directly during the first 2 nights. PSG changes over the 14 days of MJ abstinence will be reported in a separate publication.
Standard Polysomnography (PSG)
We obtained clinical PSG recordings on all participants for 2 consecutive nights following standard methodology. The bedtimes (lights out) were based on averages on the prior 5-night sleep diaries. Sleep was scored blinded to group membership in 30-sec epochs using Rechtschaffen and Kales standard criteria,18 and periodic limb movements in sleep and arousals were scored using the American Academy of Sleep Medicine (AASM) task force criteria.19,20 A sleep specialist certified by the American Board of Sleep Medicine monitored all scoring on an epoch-by-epoch basis. Sleep measures included: total sleep time (TST), sleep efficiency (SE [total sleep time/time in bed x 100]), initial sleep latency (ISL [minutes from lights out to first 30 sec of any sleep stage]), wake after sleep onset (WASO [number of minutes awake during the night after initial sleep onset]), percent total time spent in REM, stage 1, stage 2, stage 3 and 4 (slow wave sleep [SWS]), periodic leg movements with arousals (PLMA) and without arousals (PLM), and PLM and PLMA indices (average number of PLM per hour of sleep without and with arousals, respectively), and disordered breathing rate (DB = apnea + hypopnea/h). We analyzed the PSG variables for Night 1 and Night 2 separately. Clinical sleep research protocols often exclude either the first night recording or average PSG findings from Nights 1 and 2 to adjust for the “first night effect” which is an adaptation night in the sleep lab. However, since we hypothesized that sleep would become more disrupted as the length of MJ abstinence increased, we considered each night separately.
Subjective Ratings of MJ Withdrawal Craving, Mood, and Sleep Satisfaction
Withdrawal symptoms and MJ craving were measured daily after admission to the GCRC with a MJ withdrawal symptom questionnaire1 and a MJ craving questionnaire.21 Sleep quality was assessed with daily sleep logs. Psychological symptoms including mood and irritability were assessed daily with the Profile of Mood States (POMS).
Quantitative Levels of MJ (THC-COOH)
Urine collected on the day of admission and every third day was analyzed by gas chromatography/mass spectroscopy (GC/MS) for THC and 11-OH-THC, and by FPIA-fluorescence polarization immunoassay for THC-COOH. Values were corrected for urinary creatinine.
Statistical Analysis
We first conducted exploratory and normality analysis (Kolgomorov-Smirnoff) for each group separately to examine the distributional properties of the sample. When the distribution met normality assumptions, between-group comparisons (MJ users vs drug-free controls) were analyzed using independent-sample t-tests, and within-group comparisons were analyzed using paired t-tests. In the drug-free control group, the distributions of initial sleep latency, PLM index, PLMA index, and WASO were not distributed normally. Instead of transforming the nonnormally distributed data into a metric that is difficult to interpret, we chose to use nonparametric tests (Mann-Whitney). Chi-square was used to test for differences in proportion of subjects in each group with PLM and PLMA. Effect sizes (size of the between-group differences) were estimated using Cohen's d.22 To examine if there was an association between MJ use (joints/week and duration) and sleep indices, we used correlation and regression analyses.
RESULTS
Demographics
We excluded 2 MJ users and 4 controls from the final sample because their PSG studies indicated sleep apnea (AHI >10) on the PSG on either Night 1 or Night 2. Thus, the final sample was comprised of 17 MJ users and 14 drug-free controls. Table 1 presents the demographic, sleep patterns, and drug use characteristics of the groups. All the control participants and all but one MJ user were right-handed. Groups did not differ on gender, age, and mother's years of education. However, controls had higher years of education and higher estimated Shipley IQ scores (see Table 1).
Table 1.
Demographic, Sleep, and Drug Use Characteristics of the Control Group and Marijuana (MJ) Users
| Demographics | Control Group (n = 14) | MJ Users (n = 17) | t/χ2 | P |
|---|---|---|---|---|
| Age | 21.7 (3.1) [19–30] | 20.6 (2.3) [18–25] | 1.16 | Ns |
| Education | 14.4 (1.3) [14–16] | 11.5 (0.9) [9–13] | 7.19 | 0.01 |
| Mother Education | 13.4 (4.4) [12–18] | 12.3 (3.6) [10–16] | 0.75 | Ns |
| Shipley IQ | 109.5 (6.9) [100–118] | 94.9 (8.5) [85–109] | 5.17 | 0.01 |
| Gender (M/F) | 7/7 | 13/4 | 2.35 | Ns |
| Sleep History Questionnaire Prior to MJ Discontinuance | ||||
| Usual length of time in bed (h) | 7.1 (0.59) | 7.6 (0.57) | −0.69 | Ns |
| Usual hours of actual sleep | 6.5 (0.55) | 6.5 (0.51) | −0.02 | Ns |
| Sleep Satisfaction Score+ | 31 (2.6) | 29 (1.3) | 0.82 | Ns |
| Symptoms Related to Disturbed Sleep+ | 61 (8.5) | 76 (8.2) | −1.27 | Ns |
| Sleep Patterns | ||||
| Horne-Ostberg Index (% of group)F | Morningness 0 | Morningness 18 | 0.331F | Ns |
| Neither 78 | Neither 58 | |||
| Eveningness 22 | Eveningness 24 | |||
| Enter Bed (pre/post)S | 01:32/00:40 | 23:52/00:45 | ||
| Wake Up (pre/post)S | 08:00/08:12 | 08:15/07:45 | ||
| Out of Bed (pre/post)S | 08:43/08:20 | 09:20/08:10 | ||
| Drug Use Variables | ||||
| MJ use (joints/wk) | - | 104 (51) [63–210] | ||
| MJ use duration (yr) | - | 5 (3) [2–12] | ||
| Days/week MJ smoked | 7 | |||
| Alcohol average (drinks/wk) | 2 (2) [0–8] | 3 (4) [0–10] | −0.82 | Ns |
| Alcohol duration (yr) | 2 (2) [0–6] | 2 (2) [0–7] | 0.04 | Ns |
| Cigarettes (currently smoke cigarettes daily) | 1/14 | 3/17 | ||
| Average cigarette use (# cigarettes/day) | [0–1] | [0–2] | ||
| Cigarettes Duration (yr) | 1 (2) [0–4] | 3 (2) [0–6] | −1.32 | Ns |
Note: Numbers are means (SD) [Ranges]; +asked to evaluate sleep during the past few months; the higher the score, the worse the sleep quality; STimes taken from sleep logs (median values); Pre/post = admission for the control group; and MJ discontinuation for the MJ users; FFisher exact test P = 0.331.
Sleep Quality and Patterns
Based on sleep logs and the SHQ, sleep-wake patterns prior to the PSG studies did not differ greatly between the groups. The composition of the groups did not appear to be significantly different on their individual sleep pattern characteristics (i.e., time to enter bed, time to awaken, total sleep time, pre-admission and post-admission), and their morning-evening tendencies (Horne-Ostberg scale) were similar (Table 1). However, the MJ users tended to stay in bed longer after awakening than the control group both pre- and post-admission/MJ discontinuation. Additionally, no mean group differences were observed for AHI for Night 1 (drug-free controls 2.8 ± 2.5; MJ users 2.3 ± 2.2) or Night 2 (drug-free controls 3.7 ± 2.9; MJ users 3.5 ± 2.9).
The SHQ was administered to assess sleep satisfaction and the frequency of symptoms related to disturbed sleep during the past few months. This questionnaire was completed prior to the inpatient admission and therefore, before MJ discontinuation. Table 1 shows no group differences for “usual” length of time in bed or hours of actual sleep. No group difference was found for SHQ—Sleep Satisfaction (the higher the score the worse the sleep satisfaction), or SHQ—severity of Symptoms Related to Disturbed Sleep. We specifically examined the item on the SHQ that asked: “How often do you find that you use marijuana to help you sleep.” Of the 17 MJ users, answers were: never, 2; rarely, 1; sometimes, 5; often, 2; usually, 1; and almost always, 6. There was no relationship between number of joints smoked per week or duration of MJ use and those reporting using MJ “almost always” to help them sleep. That is, those who almost always smoked to help them sleep were not the heaviest users of MJ. In addition, we compared the 6 MJ users who report “almost always” smoking MJ to help them sleep to the other MJ users on Nights 1 and 2 using t-tests on all the sleep variables and found no significant group differences for any of the variables. Thus, those who “almost always” smoked MJ to help them sleep were not more likely to experience the greatest disturbance of sleep during withdrawal in the GCRC.
Although a number of our MJ users reported that they used MJ to sleep, they did not report the frequent use of other agents to help induce sleep. Seventy-seven percent of the MJ group reported that they never used alcohol to sleep (17% reported “rarely,” 6% reported “sometimes”), 11% reported rare use of sleep pills, and no MJ users reported use of medicine not including sleeping pills to sleep. For the drug-free control group, one control reported “rarely” using sleeping pills to sleep and one control reported that they “sometimes” used other medication to help them sleep. None used alcohol to sleep.
Drug Use Characteristics
We estimated MJ use (joints per week and duration) using: (1) the ASI; (2) the DUSQ; (3) the participant's self-report of the amount of money spent each week on MJ (US $2.00/joint reported by the Drug Enforcement Agency reports for the Baltimore area) and (4) self-reports of the number of joints smoked per week. We have used this same methodology in our previous work.10,11 MJ users reported smoking MJ daily (7 of 7 days), smoking a mean of 104 ± 51 joints per week (median value = 84 joints/wk), and having used MJ for a mean of 5 ± 3 years. Self-reported number of joints per week smoked was correlated with urinary THC-COOH levels on the day of admission (r = 0.75, P < 0.05). Mean MJ start age in this group was at 14 years of age (± 2 y). Both MJ and control participants reported minimal alcohol use. Furthermore, MJ and control groups did not differ in their self-reported tobacco use. Of note, our cigarette smokers were very light cigarette smokers. Only one control participant reported smoking cigarettes daily (1 cigarette per day), and only 3 MJ users reported smoking cigarettes daily (average use was 1 or 2 cigarettes per day). Our few smokers also reported low levels of addiction to nicotine on the Fagerstrom Test for Nicotine Dependence (FTND).23 The FTND medians for the 2 cigarette-smoking drug-free controls was 3 (range 0–5) and for the 8 cigarette-smoking MJ users 4 (0–7).
Statistical Analyses of Sleep Data
Despite an attempt to match groups on all the demographic measures, our groups were different on IQ and education level (Table 1). Having found that the groups differed in IQ and education, we felt that it was necessary to explore whether group differences in IQ and education could affect group differences in the sleep indices. Thus, we ran bivariate correlation analysis (Pearson) to examine the relation between these demographic variables and sleep related dependent variables. Since we found that IQ and education correlated with SWS and TST, we ran an ANCOVA to explore whether sleep related between-group differences were influenced when IQ and education were covaried. We did not correct the multiple t-tests for multiple comparisons because this pilot study is the first of its kind and we elected to use a less conservative approach.
Night 1: MJ Users and Control Group Differences in Sleep Architecture
Table 2 shows group mean differences for Night 1. MJ users had shorter TST time (t30 = 1.99, P = 0.05), less SWS min (t30 = 4.58, P < 0.001), and less SWS %TST (t30 = 3.99, P < 0.001) than controls. We report only the findings related to PLMs and exclude those related to PLMAs, since the results were similar for both measures. Although the number of leg movements was small, the MJ users had more PLMs (defined as any PLMs) than controls. Of interest, 53% of the MJ users had PLMs versus 14% of the control group. Group differences for SWS remained significant after including education as a covariate in an ANCOVA model (F1,29 = 4.95; P < 0.04). The effect sizes were moderate to large and ranged from 0.75 to 1.67.
Table 2.
Polysomnographic Measures for the Control Group and MJ Users on Sleep Nights 1 and 2
| Variable | Night 1 Control Group (n = 14) | Night 2 MJ Group (n = 17) | t/U (P level) | d# | Control Group (n = 14) | MJ Group (n = 17) | t/U (P level) | d# |
|---|---|---|---|---|---|---|---|---|
| Total sleep time (TST)(min) | 459 (42) | 420 (62) | 0.05* | 0.75 | 461 (33) | 413 (79) | 0.04*A | 0.75 |
| Sleep efficiency (TST/TIB) | 0.94 (0.05) | 0.91 (0.08) | 0.17 | 0.42 | 0.94 (0.04) | 0.89 (0.06) | 0.01** | 1.04 |
| U Initial sleep latency (ISL)(min) | 7 (11) | 16 (28) | 0.40 | 0.41 | 5 (5) | 22 (27) | 0.003** | 0.77 |
| Initial REM latency (min) | 109 (49) | 77 (70) | 0.16 | 0.52 | 116 (65) | 77 (45) | 0.04* | 0.71 |
| Slow wave sleep (SWS)/Stage 3/4 (min) | 74 (32) | 29 (22) | 0.001** | 1.67 | 70 (27) | 27 (26) | 0.001** | 1.63 |
| Slow wave sleep (SWS)/Stage 3/4 (%TST)B | 16 (7) | 7 (6) | 0.001** | 1.41 | 15 (6) | 7 (7) | 0.001** | 1.26 |
| Stage REM (min) | 99 (27) | 94 (40) | 0.70 | 0.14 | 97 (23) | 87 (30) | 0.30 | 0.61 |
| Stage REM (% TST) | 21 (5) | 23 (9) | 0.59 | 0.32 | 21 (5) | 21 (5) | 0.21 | 0 |
| UPLM index (median #/hr) | 1.5 | 2.6 | 0.07 | 2.4 | 3.4 | 0.52 | ||
| XFrequency of group with PLM (% with PLM) | 2/14 (14%) | 9/17 (53%) | 0.12 | 4/14 (29%) | 7/17 (41%) | 0.71 | ||
| UWake after sleep onset (min) | 20 (19) | 24 (21) | 0.36 | 0.19 | 22 (18) | 23 (18) | 0.63 | 0.05 |
Note. Numbers are means (standard deviations) TST = total sleep time: TIB (min): time in bed (min): PLM = periodic leg movements without and with (PLMA) arousals; U Mann-Whitney used for analyses. XChi-square used for frequency data. AGroup differences became nonsignificant after controlling for Shipley IQ score on Night 2. BGroup differences remained significant after controlling for education on Night 1 and Night 2. d# = Cohen's d was used to estimate effect size. Reference Values from the JHB sleep clinic for 20–39 year olds: total sleep time 455 (33), initial sleep latency 16 (14), wake after sleep onset 12 (8), SWS (min) >58 min = within normal limits
Night 2: MJ Users and Control Group Differences in Sleep Architecture
Table 2 shows group differences for Night 2. The MJ group showed less TST (t31 = 2.09, P < 0.05), worse sleep efficiency (t31 = 2.57, P < 0.05), longer initial sleep latency (U31 = 47, P < 0.01), shorter initial REM latency (t31 = 1.98, P < 0.05), and less SWS min (t31 = 4.32, P < 0.001) and SWS %TST (t31 = 3.51, P < 0.001). Group differences for SWS remained significant after including education as a covariate in an ANCOVA model (F1,29 = 4.88; P < 0.04). The effect sizes were moderate to large and ranged from 0.71 to 1.63. Group differences became nonsignificant for TST after including Shipley as a covariate in an ANCOVA model (F1,30 = 2.76; P < 0.44).
Comparisons of Night 1 and Night 2
For the drug-free control group, few PSG changes were evident between the two nights. In contrast, in the MJ group, mean sleep parameters were worse on the second night suggesting overall sleep was more disturbed on the second night versus the first night for the MJ group. However, none of the paired t-tests reached statistical significance. Levels of THC-COOH declined significantly from Night 1 (M = 515, SD = 826 ng/mL) to Night 2 (M = 196, SD = 261 ng/mL) (Wilcoxon signed ranks test; z = −2.85, P < 0.01). We examined the correlations of THC-COOH levels with the change from Night 1 to 2 for the various sleep variables in an attempt to address in part the issue of the extent to which the disturbed sleep is due to heavy THC use. The higher the THC-COOH level at admission, the greater the decline in TST from Night 1 to Night 2 (r = −0.733).
Subjective Ratings of MJ Craving, Mood, and Sleep Satisfaction
The MJ users completed daily MJ withdrawal symptom and craving questionnaires. The amount of MJ craving was low (a score of 40 on an 80-point scale), changed little over time (morning after Night 1-Day 1; M = 40, SD = 18; morning after Night 2-Day 3; M = 37, SD = 15), and did not correlate with any of the PSG measures. Likewise, the total withdrawal symptom score did not change from Day 1 (7.6 ± 3.6) to Day 2 (7.5 ± 5.2) and did not correlate with any PSG measures. In addition, the MJ users did not endorse items reflecting increases in depressed mood or irritability on the POMS over the 3 days of abstinence.
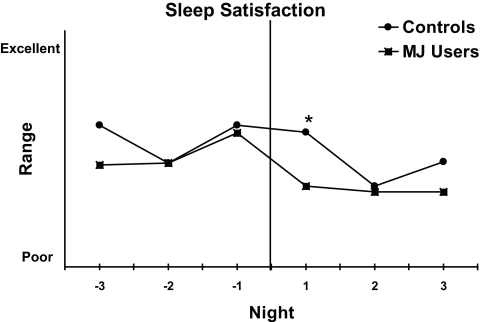
All participants rated their Sleep Satisfaction (sleep diary) for 5 mornings “pre” and 3 mornings “post” admission/MJ discontinuation. Group differences in sleep satisfaction were only found on Night 1 (t29 = 2.10, P < 0.05; Figure 1). However, the MJ group reported less sleep satisfaction than controls for all nights with the exception of Night −2 when the groups rated sleep satisfaction equally. Within-group comparisons showed a decline in sleep satisfaction from the last night prior to MJ discontinuation (Night −1) to the first night after MJ discontinuation (Night +1) t12 = 2.14, P < 0.05, in the MJ users but not the drug-free controls suggesting that withdrawal symptoms may have contributed to their subjective reports.
Figure 1.
Sleep satisfaction from 3 mornings pre-discontinuation of MJ or pre-admission to the GCRC and 3 mornings post-discontinuation or post-admission. The vertical line represents MJ discontinuation. * indicates group differences on +1 Night. The MJ group reported significantly less sleep satisfaction on Night +1 of MJ discontinuation than on Night −1 prior to MJ discontinuation (P < 0.05).
Relationship between MJ Use (Joints/Week and Duration) and Sleep Architecture
In the MJ group, we used linear regression analyses and included joints/week, joints/week squared, duration and duration squared, as independent variables and only the sleep variables with normal distributions for both nights as the dependent variables into the models. We found no significant associations between joints per week and duration of MJ use and any of the sleep related indices. We also examined if the 5 MJ users meeting the diagnosis for cannabis dependence showed more sleep disturbance than those MJ users not meeting diagnostic criteria. No group mean differences were detected on any of the sleep variables using t-tests.
DISCUSSION
The MJ users showed differences in PSG measures (lower total sleep times, and less slow wave sleep than the control group) on both nights; they also showed worse sleep efficiency, longer sleep onset and shorter REM latency than the control group on Night 2. More sleep continuity parameters were significantly worse for the MJ group than the control group on Night 2 versus Night 1, indicating that sleep in the MJ group was relatively worse on Night 2 compared to Night 1. Of note, the MJ group did not show improved sleep after an adaptation night as expected. The effects were moderate to large. Withdrawal symptoms, craving, and depression did not appear to influence these findings.
During the 2 nights after MJ discontinuation, the MJ users had less total sleep time, lower sleep efficiency, longer sleep latency, shorter initial REM latency, and less SWS (min and %TST). Although not reaching significance statistically, perhaps due to the small sample size and intersubject variability, the MJ users tended to show less REM (min), more PLMs, and more WASO. Sleep disruption appeared to become worse on Night 2 compared to Night 1 after MJ discontinuation. This is in contrast to the typical sleep laboratory finding of improved sleep after an adaptation night and thus could be related to the effects of decreasing concentration of THC-COOH on sleep.
We did not find any association between amount or duration of MJ use and any of the sleep variables. This was somewhat surprising since we have shown an association between the number of joints per week of MJ smoked and neurocognitive functioning10 and brain activity during specific tasks.11,24 We speculate that specific aspects of sleep behave differently than specific areas of neurocognitive functioning. This needs to be explored further in a larger sample of MJ users with a wide range of MJ use. In future studies, we plan to extend our investigation of the relationship between amount and duration of MJ use and sleep disturbances and include an examination of daily patterns of use. For example, the subset of MJ users who only smoke MJ prior to bedtime may be the subset of MJ who show the most sleep disturbance.
In general, young adults show abundant levels of SWS (deep sleep), averaging greater than 58 min during the night and about 20% of TST. Subjective reports of disturbed sleep in MJ users once they discontinue MJ use1,2 may relate to lower levels of SWS.25 Decreased SWS has previously been reported in abstinent MJ users26 and chronic alcoholics; SWS can often take more than 6 months to recover to normal amounts.27,28 For Nights 1 and 2, the MJ users had shorter mean initial REM latency than controls. A shortening of initial REM latency (< 90 min) may be secondary to a rebound phenomenon that may be related to reports of REM suppression from acute MJ administration.5,6
This pilot study is one of the few studies that examine PSG characteristics with discontinuation of heavy MJ use. The MJ users consistently showed more sleep disturbance than the drug-free control group. Since no PSG data were collected during the period prior to discontinuation of MJ use, we can not discern whether the disturbed sleep findings reflect general differences between MJ users and drug-free controls, or are related to cessation of MJ use.
A strength of our study was that both groups were similar in baseline demographics and sleep characteristics based on sleep logs and SHQ measures of sleep habits. Therefore, we believe that some degree of the difference seen between the groups is related to the use and discontinuation of MJ. While there were more men than women in the MJ group, additional analyses found no sex-related differences on any of the PSG measures and therefore would not have influenced these findings. In addition, alcohol intake and nicotine use was minimal in both groups, and the MJ group reported using only MJ. We also excluded individuals with current or past dependence on any other substance or if their urine toxicology screens were positive for drugs other than MJ. Furthermore, these effects are unlikely related to comorbid mood or personality alteration, since we excluded MJ users with comorbid Axis I psychiatric disease and antisocial personality disorders, as well as any physical or neurological disorder that may affect sleep. Since there was little change in ratings of withdrawal symptoms, craving, or mood, we do not believe that the observed sleep disturbance was psychologically/craving induced. This is likely because our participants withdrew from MJ in an inpatient setting where environmental cues that elicit craving are absent. Rather, we postulate that the detected sleep disturbance in MJ users is more likely associated with alterations of the neural substrates of sleep.
The association between the use and cessation of MJ and sleep disturbance is biologically plausible and we believe that there are neurobiological mechanisms to explain such a relationship. Marijuana's primary active constituent is delta-9-tetrahydrocannabinol (Δ9-THC) with neural effects mediated through abundant cannabinoid (CB1) receptors in the brain. One of the effects of THC administration is sedation. Our group of MJ users confirmed this, as many of them reported on the SHQ that they use MJ to help them sleep. Interestingly, the MJ users report negligible use of alcohol, sleeping pills, or other medicines to induce sleep. Proposed mechanisms for this action have included reports that endogenous cannabinoids increase adenosine (a sleep promoter)9 and that CB1 mRNA is co-expressed with neuropeptides of the lateral hypothalamus resulting in inhibition in arousal systems.9 Thus, the examination of sleep disturbance in heavy MJ users increases our knowledge about cannabinoids influence on sleep.
The use and discontinuation of MJ use and disorders of sleep may involve similar brain regions.11,24,29–31 The prefrontal cortex (i.e., anterior cingulate cortex, dorsolateral prefrontal cortex, and the orbitofrontal cortex) plays an important role in normal sleep and alterations in this region are reported in persons with insomnia,30 sleep deprivation,29 and in 30-day abstinent heavy MJ use.11 The orbitofrontal cortex is a brain region of special interest. Discontinuation of MJ use and difficulty initiating and maintaining sleep are associated with decreased metabolism in the OFC24,30 and acute administration of THC increases OFC metabolism,32 which may alleviate insomnia. This mechanism may explain the propensity to relapse after a short abstinent period.
Although these findings are in a small sample of MJ users, our results are robust and biologically plausible. Effect sizes were medium to large according to Cohen (> 0.50). In addition, we selected participants stringently and were able to match the groups on a number of important variables including morningness-eveningness traits, overall ratings of sleep quality, and sleep pattern characteristics. Nevertheless, the present data cannot determine where MJ use and sleep disturbance fall in the causal pathway. For example, it is possible that individuals with innate sleep problems in early childhood and adolescence are more likely to abuse illegal substances and alcohol later in life.33,34 If our sample of MJ users had innate sleep problems then it is interesting that they report using only MJ to help them sleep and not other substances including alcohol, sleeping pills, and other sleep inducing formulations. Other limitations of the study include limited generalization to all users of MJ since our sample was primarily young, reported sleep disturbance when discontinuing smoking MJ in the past and some smoked large amounts of MJ. We selected only MJ users who reported sleep disturbance when attempting to discontinue MJ use in the past because as a first step, we were only interested in our ability to determine if objective PSG abnormalities were present in a carefully chosen sample of MJ users self-reporting sleep disturbance with discontinuation of MJ use. Also, because our far-reaching goal is to determine the clinical significance of sleep disturbance on treatment outcome in MJ users, we focused our efforts only on MJ users reporting sleep disturbance during past attempts at abstinence.
From an addiction treatment perspective, it is not critical whether sleep disturbance precedes or follows MJ discontinuation, but rather if disturbed sleep precipitates relapse in treatment-seeking MJ users. If disorders of sleep contribute to relapse of MJ use, then we could treat MJ users experiencing sleep difficulties with appropriate behavioral and pharmacological approaches. Ameliorating some of the unpleasant withdrawal symptoms would likely increase the number of heavy MJ users who successfully complete drug rehabilitation. Many questions related to sleep disorders in substance abusers remain unanswered highlighting the importance of further investigation on this important topic.
ACKNOWLEDGMENTS
We thank the nurses and clinical staff at NIDA-IRP and the Johns Hopkins Bayview GCRC who contributed to this project. We especially thank Debra Hill, BA, for computer and database support.
Supported by NIH grants DA 17122(KB), the JHBMC-GCRC (MO1 RR02719) and the DHH NIDA Intramural Research Program.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Lesage has participated in speaking engagements for GlaxoSmithKline. Dr. Neubauer has been a consultant to and has participated in speaking engagements for Sanofi-Aventis and Takeda and has consulted for Neurocrine Biosciences and Pfizer. Dr. Funderburk has been a consultant/statistician for Nova Flux Technologies and is the owner of InCompass Systems, an independent research and consulting company. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Budney AJ, Moore BA, Vandrey BS, Hughes MD. The timecourse and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- 2.Kouri EM, Pope HG. Abstinence symptoms during withdrawal from chronic marijuana abuse. Exp Clin Psychopharmacol. 2000;8:483–92. doi: 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- 3.Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. JAMA. 2004;291:2114–21. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- 4.Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004;161:1967–77. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- 5.Feinberg I, Jones R, Walker J, Cavness C, Floyd T. Effects of marijuana extract and tetrahydrocannabinol on electroencephalographic sleep patterns. Clin Pharmacol Ther. 1976;19:782–94. doi: 10.1002/cpt1976196782. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg I, Jones R, Walker JM, Cavness C, March J. Effects of high dose delta-9-tetrahydrocannabinol on sleep patterns in man. Clin Pharmacol Ther. 1975;17:458–66. doi: 10.1002/cpt1975174458. [DOI] [PubMed] [Google Scholar]
- 7.Liguori A, Brown TW, McCall W. Sleep architecture changes during marijuana withdrawal. Sleep. 2004;27:54–55. [Google Scholar]
- 8.Haney M, Hart CL, Ward AS, Foltin RW. Nefazodone decreases anxiety during marijuana withdrawal in humans. Psychopharmacology. 2003;165:157–65. doi: 10.1007/s00213-002-1210-3. [DOI] [PubMed] [Google Scholar]
- 9.Murillo-Rodriguez E, Blanco-Centurion C, Sanchez C, Piomelli D, Shiromani PJ. Anandamide enhances extracellular levels of adenosine and induces sleep: an in vivo microdialysis study. Sleep. 2003;26:943–7. doi: 10.1093/sleep/26.8.943. [DOI] [PubMed] [Google Scholar]
- 10.Bolla K, Brown K, Eldreth D, Tate K, Cadet J-L. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–43. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 11.Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–92. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Smith SS. Addictive drug survey manual. Baltimore: NIDA Addiction Research Center; 1991. [Google Scholar]
- 13.McLellan AT, Luborsky L, Woody GE, et al. An improved evaluation instrument for substance abuse patients: the Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Robins LN, Helzer JE, Croughan J, et al. National Institute of Mental Health Diagnostic Interview Schedule: Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–9. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 15.Zachary RA. Shipley Institute of Living Scale: Revised Manual. Los Angeles: Western Psychological Services; 1991. [Google Scholar]
- 16.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 17.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 18.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stage of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 19.Bonnet M, Carkey D, Carskadon MA, et al. EEG arousal: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 20.Atlas Task Force of ASDA. Recording and scoring leg movements. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 21.Heishman SJ, Singleton EG. Assessment of cannabis craving using the Marijuana Craving Questionnaire. Methods Mol Med. 2006;123:209–16. doi: 10.1385/1-59259-999-0:209. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 23.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 24.Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–20. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 25.Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: Evidence for occult insomnia. Drug Alcohol Depend. 2006;82:238–49. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Freemon FR. The effect of chronically administered delta-9-tetrahydrocannabinol upon the polygraphically monitored sleep of normal volunteers. Drug Alcohol Depend. 1982;10:345–53. doi: 10.1016/0376-8716(82)90036-9. [DOI] [PubMed] [Google Scholar]
- 27.Ishibashi M, Nakazawa Y, Yokoyama T, et al. Cerebral atrophy and slow wave sleep of abstinent chronic alcoholics. Drug Alcohol Depend. 1987;19:325–32. doi: 10.1016/0376-8716(87)90019-6. [DOI] [PubMed] [Google Scholar]
- 28.Williams HL, Rundell OH., Jr. Altered sleep physiology in chronic alcoholics: reversal with abstinence. Alcohol Clin Exp Res. 1981;5:318–25. doi: 10.1111/j.1530-0277.1981.tb04905.x. [DOI] [PubMed] [Google Scholar]
- 29.Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends Cogn Sci. 2002;6:475–81. doi: 10.1016/s1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- 30.Drummond SP, Smith MT, Orff HJ, Chengazi V, Perlis ML. Functional imaging of the sleeping brain: review of findings and implications for the study of insomnia. Sleep Med Rev. 2004;8:227–42. doi: 10.1016/j.smrv.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Chuah YM, Venkatraman V, Dinges DF, Chee MW. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. 2006;26:7156–62. doi: 10.1523/JNEUROSCI.0906-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volkow ND, Gillespie H, Mullani N, et al. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res. 1996;67:29–38. doi: 10.1016/0925-4927(96)02817-x. [DOI] [PubMed] [Google Scholar]
- 33.Johnson EO, Breslau N. Sleep problems and substance use in adolescence. Drug Alcohol Depend. 2001;64:1–7. doi: 10.1016/s0376-8716(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 34.Wong MM, Brower KJ, Fitzgerald HE, Zucker RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcohol Clin Exp Res. 2004;28:578–87. doi: 10.1097/01.alc.0000121651.75952.39. [DOI] [PubMed] [Google Scholar]