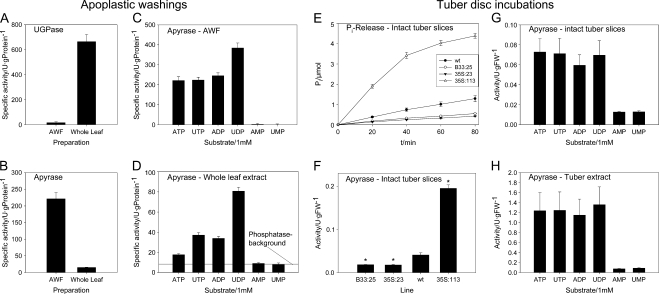
Figure 7.
Apyrase activity in apoplastic washings and in tuber slices in incubation experiments. A to D, To investigate the subcellular localization of apyrase, the specific activities of apyrase (B; 1 mm ATP as substrate) and of the cytosolic marker enzyme UGPase (A) were determined in AWF obtained by centrifugation of infiltrated wild-type potato leaves and compared with the activities assayed in whole leaf extract obtained from the same plants. The substrate specificity of apyrase/5′-nucleotidase was assayed in both AWF (C) and whole leaf extract (D) using 1 mm ATP, UTP, ADP, UDP, AMP, or UMP. The horizontal line indicates the level of nonspecific phosphatase activity on the basis of nucleoside monophosphate hydrolysis. E to H, Experiment with tuber slices. Freshly prepared and washed tuber slices of the wild type, B33-RNAi line B33:25, 35S-RNAi line 35S:23, and 35S overexpressor line 35S:113 were incubated with 3 μmol of ATP in 3 mL of buffer in a final concentration of 1 mm to determine apoplastic apyrase activity as release of Pi from ATP in a time course (E) or in absolute activities per gram fresh weight (FW; F). The substrate specificity of apyrase/5′-nucleotidase in intact tuber slices (G) and in total tuber enzyme extracts produced from wild-type plants using substrate concentrations of 1 mm (H) are also shown. Results are means ± se (n = 6–8). In F, significant differences from the wild type according to Student's t test are indicated with asterisks (P < 0.05).