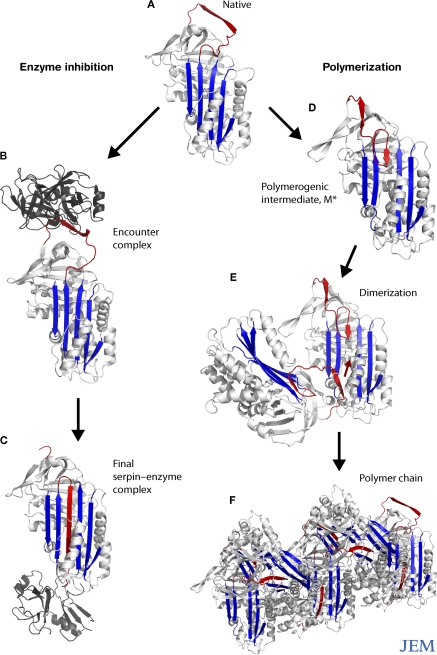
Figure 1.
Conformational transitions of the serpins. The reactive loop of α1-antitrypsin (A, red) binds to neutrophil elastase (B, dark gray), triggering a conformational change that flips the enzyme from the apex to the base of the serpin (C). This is associated with insertion of the reactive loop of the target proteinase as an extra strand into β-sheet A (blue) and inactivation of the catalytic triad of the protease. Point mutations subvert this mechanism and cause aberrant conformational transitions (D) and the formation of polymers (E and F).