Ever since I first fell under the intellectual and emotional sway of biomedicine some 30 years ago I have been struck by the beauty and power of developmental biology. That a fertilized egg divides progressively and, via the directions of some mysterious cell-intrinsic genetic program, differentially expresses genetic programs in distinct daughter cells that results in organogenesis, is nothing short of miraculous. What hematologists perceived and gradually articulated is that organisms continue to take advantage of these same developmental cellular and genetic programs beyond embryogenesis, and in fact throughout life, for tissue renewal, regeneration and repair. The appreciation of this axiom—the notion of tissue-specific adult stem cells-also arose in studies of the gastrointestinal epithelium, muscle and bone, and grew to include all areas of developmental histology. It is the universality of this principle that drives much of the intellectual excitement and translational hope for studies of stem cell biology in the 21st century (1).
One of the key features of tissue-specific stem cells, as posited in theory and validated by experiment, is self-renewal. That is, when stem cells divide, at least some of the daughter cells must become stem cells themselves, with a (presumably long) lifespan identical to or similar to that of the original parent stem cell. During development with the dramatic expansion of individual cellular compartments, tissue-specific stem cell numbers increase, implying that somewhat more than 50% of all daughter cells produced by dividing stem cells become stem cells. Once the organism reaches its mature size, however, stem cell divisions would appear to be roughly balanced, with 50% of daughter cells differentiating and 50% becoming stem cells, thus supporting stable stem cell numbers and tissue regeneration over years.
A related notion to asymmetric but balanced stem cell division is that self-renewal occurs predominantly, if not exclusively, within tissue-specific stem cell compartments. Within the hematopoietic compartment, for example, only the most primitive hematopoietic stem cells self-renew, while it appears that more developmentally mature cells fail to self-renew upon division. Instead, all daughter cells produced are themselves more differentiated. In fact, leukemias are fundamentally described by deregulated self-renewal occurring at all stages of maturation, resulting in greatly expanded intermediate-stage precursor compartments.
But is self-renewal truly limited to tissue-specific stem cells? In the same sense that developmental genetic programs are used throughout life, might physiology inducibly activate stem cell programs under precise circumstances in non-stem cells, to allow for the expansion and persistence of specific committed cells? Such an ability to reactivate stem cell genetic programs might be most useful in the immune response, where antigen-specific T and B cell responses must be induced, expanded and maintained throughout months and years.
Given our longstanding interest in both stem cell biology and transplantation immunology, we hypothesized that the answer is “yes,” and we have searched for “memory stem cells” in allogeneic T cell responses in vivo. Several physiologic clues have led to this hypothesis. First, specific immune responses must be longlived, and yet end-stage immune effector cells apoptose. Second, immune responses are much harder to treat (once they are already activated) than to prevent. Therefore, somewhere there must be long-lived cells, that self-renew and sustain immune responses, an immunologic stem cell.
The model system that we have used to study this problem is allogeneic immune (graft-versus-host) responses following hematopoeitic stem cell transplantation (SCT). In these systems, donor CD8 T cells are activated on host antigen-presenting cells, and mount a sustain attack on host tissues. This reaction, when mild and contained, can effectively destroy residual diseased stem cells in, e.g., patients with leukemia. But when severe and widespread, this reaction can attack all the normal host tissues. This “graft-versus-host disease” causes severe morbidity and can lead to patients' death.
Graft-versus-host alloimmune responses are initiated by host-specific CD8 T cells (2). These antigen specific cells are generated from a precursor population of post-thymic, mature T cells that are antigenically naïve, and which express low levels of the protein CD44 and high levels of the protein CD62L on their cell surfaces. During the process of antigen exposure and activation, either in vivo or in vitro, CD44 is upregulated, and then CD62L is down-modulated (3). While Yi Zhang, a very talented senior postdoctoral fellow in my laboratory, was studying a mouse model of human hematopoietic stem cell transplantation, looking for late developments in the pathogenesis of graft-versus-host-disease, we noticed that, even several months after a SCT, a significant fraction of donor CD8 T cells had the phenotype of naïve T cells, While this mouse model was not transgenic, and only a minority of the T cells would be expected to be antigen reactive, the number of such persistent CD44loCD62Lhi CD8 T cells seemed to be far too high, and we took a closer look. And we were immediately pleasantly surprised, when we learned that all of these “naïve” cells had actually divided at least 7 times since transplantation into the host mice, hardly the reaction of a naïve T cell. Subsequent experiments showed that an extremely large fraction of these “naïve” phenotype cells—perhaps as many as 20–40%-were Ag-specific T cells which recognized host tissue antigens. So, what were these cells, and what could they do physiologically?
First, we asked whether the cell surface phenotype of these cells was identical to, or distinct from, truly naïve CD8 T cells. Like naïve cells, these post-mitotic CD44loCD62Lhi cells were CDllb-, CD127-, and did not produce either IFNg or granzyme B. However, unlike naïve CD8 cells, they expressed significant levels of CD122 and high levels of the stem cell antigen Sca-1. In addition, they expressed detectable mRNA signals for the chemokine receptors CCR7 and CCR5, which naïve CD8 T cells do not.
When these post-mitotic cells were placed into culture with host dendritic cells, they proliferated rapidly and extensively, in fact far more so than cannonical CD44hi effector, effector memory or central memory subsets. Thus, this novel population indeed contained highly proliferative, host-antigen specific cells. In addition, when the cultured cells recovered and restained with CD44 and CD62L, it was clear that CD44 hi effector/effector memory and central memory CD8 T cells were regenerated from these post-mitotic CD44loCD62L hi cells. Perhaps even more exciting, additional CD44loCD62Lhi cells were produced during this proliferation. Thus, this new population showed the ability to self-renew and produce more mature antigen specific cell compartments upon re-exposure to alloantigen in vitro, suggesting that it had the physiologic properties of an antigen specific memory CD8 T cell.
Yi went on to show that this same process of self-renewal and recreation of all antigen-specific T cell compartments also occurred if these memory CD8 T cells were purified and retransplanted into a secondary recipient of the same strain as the original recipient. What was more, these secondary recipients suffered the signs of GVHD, just as if they had been injected with large numbers of antigen-specific effector T cells. Taken together, these results suggested that these post-mitotic, antigen-specific CD44loCD28L CD8T cells were the self-renewing reservoir of the CD8 T cell response, functioning as true tissue/function specific T cells (4). Subsequent work has shown that such CD8 memory stem cells are created during many, and perhaps all, chronic immune responses, including anti-viral responses.
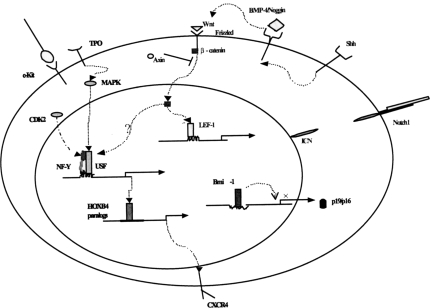
While very interesting from the point of view of immunology, the overarching lesson is perhaps of more general interest. These results imply that stem cell physiology can be reactivated in non-stem cells, resulting in self-renewal and expansion of specific long-lived compartments in specific locales for specific functions. Such reactivation is not a malignant property per se, but is a normal regulated function, at least within the immune system. Does this reactivation of stem cell physiology use the same molecular pathways as do hematopoietic or embryonic stem cells? Are BMP and Wnt pathways involved? Bmi-1? Notch, alone or in tandom activation with MAPK (5)? (Figure 1) We do not yet know, but the answers to these questions will be the subject of future experiments, and the basis for exciting new therapeutics, in the years to come.
Fig. 1.
Signal Transduction Pathways Implicated in Stem Cell Self-Renewal. In the hematopoietic and embryonic stem cell systems, overexpression and gene targeting studies have implicated many signal transduction pathways, including Notch/jagged, Wnt/LEF-1, NF-Y/Hox, MAPK, and Bmi-1. Each of these pathways, or perhaps other novel pathways, could be activated in lineage committed cells such as CD8 memory stem cells, to stimulate self-renewal at later stages of differentiation.
DISCUSSION
Mackowiak, Baltimore: Steve, congratulations. It's exciting. Thank you for sharing that with us. Could you tell us a little bit about stem cell senescence?
Emerson, Philadelphia: That is actually a very subtle question, and we don't know all the answers. You would think from the way that we frame our notion of stem cells as self-renewing, that they wouldn't senescence; rather, that they could produce daughter stem cells permanently. However, we don't think that's true and one of the first clues of this came from studies of clonality in patients with hematologic diseases. It turns out that you can't really count effectively the number of hematopoietic stem cells in young individuals. There are too many, certainly over 100,00 functional stem cells. But if you look in adults over the age of 60, I don't want to scare anybody, but there are probably less than 20 functional stem cells left working at any one time. This data comes from studies of androgen receptors skewing in women whose X chromosomes have been Lyonized early on in life. Since the number of stem cells that are active declines throughout life there must be senescence. We are not yet certain what the key pathways are that control that. It is probably related to an INK4 expression and something to do with telomer length control, that but this is totally a new area that we are just starting to interrogate.
Powell, Galveston: My question is, do these stem cells have expressed multi-drug resistance genes so they will be hard to ablate with therapy?
Emerson, Philadelphia: Absolutely, the answer is yes. So when the MDR genes were first discovered, they were thought to be somehow just tumor specific. But, in fact, they are not and that is probably why these MDR genes exist. They are pumps which they extrude dyes, phytotoxins from mushrooms and other potential natural pathogens. So there is an evolutionary survival benefit to having one's stem cell spools express high levels of MDS, and they do. All stem cells express high levels of MDR, they are, in fact, isolatable by virtue of high expression of MDS. So that is probably why it is so difficult to eradicate tumors. If you look at any tumor collection, 99.9% of the cells are pre-apoptotic anyway, but it's the highly expressive MDR positive subset that is most resistant.
Jordan, Los Angeles: Very intriguing information. For those of us in autoimmunity and transplant, I think it is obviously quite interesting. My question for you is, do these cells express CD4, CD8? Would they have targets that could respond to T-cell depletional therapies which may make autoimmune diseases better or prevent rejection, and if so, how do they regenerate, or how do they become activated? Can you measure that, for example, by looking at intracellular gamma interferon in response to a specific antigen? How do you identify them?
Emerson, Philadelphia: In this system, these memory stem cells are CD8 cells. We believe they exist also in the CD4 compartment. What is interesting about it is that the original stem cell model in hematopoiesis said it was only the first cell that had stem cell capabilities, but these cells acquire the cell familial capability after they have seen antigen. It is an acquired phenotype, and so it is very interesting, and we are just now learning what activation pathways are involved, when they see antigen and when the subcompartment is created, and that is what we are working on because that will give us ways to intervene. This is a very important problem. It turns out, you can blunt or you can prevent almost any immune reaction up front with cyclosporine and many other ways, but once activated, T-cells are much more resistant.
Tweardy, Houston: I enjoyed your talk. I guess the question I have is the potentiality of the cell that you have identified. Is it capable of recapitulating, as you say, when transplanted, graft versus host, which is a CD8 T-cell response. Does it have any other capability in terms of transferring to a CD4, B-cell, dendritic cell or any other compartment of the immune system?
Emerson, Philadelphia: Is it really a stem cell, or is it a committed pregenerator for one lineage? That is a really good question, and our presumption is that it is actually a committed stem cell for single lineage. Alternatively, it may be that there is some plasticity there that we need to find out about. In fact, there is data claiming that, it comes from Russ Kaufman at Duke, that if you take T-cells and you culture them, you can actually get hematopoietic cells at low frequency, red cell precursors and so I am not sure that is so, but the question is still open.
Sacher, Cincinnati: My question actually is a sort of follow up of yours in many ways, because obviously we in jargon refer to stem cells as being pluripotent or multipotent, you know capable, undifferentiated or differentiated, and clearly it is the micro-environment that must play a role in this. My question to you is, is there any evidence that some of these cells may be differentiated?
Emerson, Philadelphia: Yes, but not to the extent that you are asking. We think that this memory stem cell compartment actually derives both from a totally naïve cell and also from a bit more differentiated cell to develop in the first week of the immune response. And actually, the pool develops by de-differentiation of the activated T-cells early on. The data suggests that the immune system gets revved up and the acute response starts, and actually, if you block it there, you don't generate these stem cells. Now whether or not those cells have more than one lineage capacity afterwards, as I said, we are not sure yet.
Jordan, Los Angeles: Just quick question. Do you know if these cells are influenced by regulatory key cells, CD25? Can they be down-regulated by them?
Emerson Philadelphia: We don't know.
REFERENCES
- 1.Emerson S.G. Hematologists and the Stem Cell Revolution. The Hematologist. 2006;3:1. [Google Scholar]
- 2.Shlomchik W., Couzens M.S., Tang C.B., McNiff J., Robert M.E., Liu J., Shlomchik M.J., Emerson S.G. Prevention of Graft Versus Host Disease by Inactivation of Host Antigen-Presenting Cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y., Joe G., Hexner E., Zhu J., Stephen G., Emerson Dendritic cell-activated CD44hiCD8+ memory T cells are defective in mediating acute graft-versus-host disease but retain graft-versus-leukemia activity. Blood. 2004;103:3970–3978. doi: 10.1182/blood-2003-09-3135. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y., Joe G., Hexner E., Zhu J, Emerson S.G. Post-Mitotic CD44lo CD8 T Cells in GVHD are Memory Stem Cells. Nature Medicine. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 5.Stein M.I., Zhu J., Emerson S.G. Molecular Pathways Regulating the Self-Renewal of Hematopoietic Stem Cells. Experimental Hematology. 2004;32:1129–1136. doi: 10.1016/j.exphem.2004.08.012. [DOI] [PubMed] [Google Scholar]