Abstract
In the water column, planktonic copepods encounter small-scale hydrodynamic disturbances generated by fellow zooplankters. Our question is whether or not the copepods can distinguish between hydrodynamic disturbances created by predators, prey, conspecifics and/or mates. We used a Schlieren optical system with a density gradient in the water volume and filmed at 48 frames per second to record the behaviour of copepods during encounters with an artificial hydrodynamic disturbance. We observed the reactions of Cyclops scutifer and Epischura nordenskioldi towards disturbances of different strengths. We also re-examined an earlier report on tandem swimming in C. scutifer while attempting to mate, using novel mathematical tools to analyse possible correlations between the two mates. We conclude that the information within the hydrodynamic disturbances created by swimming zooplankters has enough content for differentiated reactions. We also suggest that the adaptive value of tandem swimming during mating results in offspring capable of executing escape reactions comparable in strength to the disturbances.
Keywords: Schlieren; deformation of fluid; mechanoreception; escape reaction, mating; footprints
1. Introduction
Zooplankton—the community of animals floating in water volumes, which swim at speeds and turn so often that they cannot get from one defined location in space to another defined one as, for example, migrating birds and fishes can—forms an important component of the aquatic and marine food web. Most, if not all, members of this community move very slowly and/or are very small in size. Their Reynolds numbers, the ratio of inertia to viscosity, are low, making viscosity an important factor in the lives of these animals. The Reynolds number of each animal is determined by one characteristic of water, the kinematic viscosity, and two characteristics of the animal, its size and speed. Speed is a result of the animal's behaviour and physiology and can, therefore, change within a short time. The animal's size and the kinematic viscosity of the surrounding water stay constant for at least some time. Our first question is whether or not planktonic animals change their swimming behaviour constantly in order to either save energy or to modify the disturbances they create while swimming. By changing the disturbances, they could achieve higher encounter rates with mates, for example. Alternatively, they could zero-in on an averaged best solution, a kind of behaviour that fits all situations. Our second question is how is the behaviour maintained from generation to generation and over the millions of years these animals have populated the ponds, lakes and oceans?
Here, we report the results we have obtained over a period of 30 years while studying the swimming behaviours of freshwater planktonic copepods. The size of these animals, 0.1–0.5 cm, and the speed with which they swim through their three-dimensional media, 0.1–1 cm s−1, challenged the experimenter. In order to observe their behaviours and register the transitions from one pattern to the next, we had to use optics that allowed viewing the appendages and their motions (e.g. Costello et al. 1990). However, in this case, the animal had to be tethered, not free-swimming. Free-swimming animals need space, rendering them as small objects on a video encompassing the whole vessel. In addition, when an animal executes an escape reaction, its speed reaches 20–100 cm s−1 for a very short time (e.g. Buskey et al. 2002; Buskey & Hartline 2003). Therefore, simple video recordings will render some information, but the amount will be too small to understand the copepod's actions while swimming around. Realizing that one needs both free-swimming animals in a large volume of water and the evidence of the animals' behaviours even when they move at high speeds, Strickler resorted to the optical design of Schlieren photography (Toepler 1866; for details see Strickler 1975a,b, 1977).
2. Approach
In the Schlieren optical pathway, the vessel containing a slight density gradient is an optical element of the system. Any local change within this density gradient will be visible. Copepods, and other zooplankters of similar size, swimming within this density gradient will modify the gradient according to their behaviour. They swim within a Reynolds number range of approximately 1–10 and, therefore, the boundary layers around their bodies and all moving appendages are relatively large (for calculations see Vogel 2003, p. 166). Hence, they will carry minute volumes of water from the former density layer with them when they move even small distances to a new density layer. Figure 1 exemplifies the results from this technique. A zooplankter, a helmeted Daphnia lumholtzi (Cladocera: Crustacea), moved from one position to the next and, at each position, pumped a volume of water through the carapace in search of food.
Figure 1.
Schlieren observation of swimming helmeted D. lumholtzi. The animal entered the observation volume from the lower right and with four hops reached the position in the mid of the picture. It pumped a volume of water through the carapace to feed and, since there was little food, it hopped twice to the new position at the upper left. There it tried to feed again. Duration for this path was approximately 6 s. Scale bar, 0.1 cm.
Observing many pictures similar to figure 1 gives the impression that these zooplankters use their ‘physique’ and the physics governing the flow of water around them to ‘talk’ with their fellow zooplankters, to send information in order to find mates and to receive information from prey and predators (Strickler 1975b). This notion generated the next question of whether or not different species disturb the water in different ways and if so, are there species-specific patterns visible. We called these patterns ‘footprints’ to stir the reader's imagination pointing to a possible analogue to footprints in fresh snow created by animals such as rabbits, deer and foxes, leaving identifying information behind them. Our observations show that since the zooplankters have different species-specific morphologies, they disturb the water in recognizably different ways (Kerfoot et al. 1980; Yen & Strickler 1996).
Considering that these animals create distinct footprints, the next question would be: do these animals recognize each other's footprints? And if so, do they show modulations of their reactions towards stronger or weaker footprints? Does a predator animal react differently than its prey towards the same footprint? Additionally, when adding mating to the palette of behaviours, the following question arises: are footprints involved in finding a mate?
The question about modulation of the reaction is an important one. Free-swimming, self-propelled animals disturb the water (e.g. Jiang & Osborn 2004). When an animal encounters such a disturbance which was generated by its neighbour, it most probably exerts an escape reaction (e.g. Strickler 1975b, Buskey et al. 2002). For mating, where a male and a female must meet and, for a while, be in close contact, inducing an escape reaction would have a negative effect. So, besides expecting a different reaction towards a more- or less-strong footprint, and a different reaction from a predator than from a prey, one would argue that in the mating behavioural sequence, the male must follow the female, but at such a distance that does not trigger an escape on her part, and yet, still perceives her position in order to follow her.
3. Visually recording copepod behaviours
Claus (1863, p. 84) described the morphologies of different copepod species and added some remarks about their swimming behaviours. He observed that many calanoid species remained almost motionless at the same spot in the water while they created a feeding current with their mouthparts. He added that from time to time these animals dashed very fast to a new spot and then resumed their feeding behaviour. Further, he pointed out that cyclopoid copepods differed; they swam in a ‘hop-and-sink’ pattern using their swimming legs once per hop. They did not create a feeding current. That these animals migrate considerable vertical distances within the water column, being close to the top at night and in the deep during the daytime, had been mentioned earlier by Cuvier (1829). Therefore, one can assume that these free-swimming animals will encounter each other during the time period of a day. However, for ca 100 years, most of the research centred on the important ecological role these zooplankters play within the food-web of oceans and freshwater bodies, and little effort has been extended to illuminate how these animals interact with each other.
Strickler (1970) aimed at finding out what signal stimulates male Cyclops abyssorum praealpinus to reverse their downward swimming in the early afternoon and start swimming towards the surface of the water column. In simultaneously observing over 50 animals reacting to changes in light intensities, an additional result ensued. Despite a high probability of encountering each other, the animals avoided their nearest neighbours by adjusting their swimming directions. This observation gave rise to two questions. Question one: what are the sensors involved? Strickler & Bal (1973) used high-speed photography as well as electron microscopy to show that mechanoreceptors on the antennules may transmit the signals left by swimming neighbours when their motion generated deformations of the fluid, such as strain rate and vorticity. Question two immediately followed: how can we mimic a swimming neighbour and cheaply record the swimming performances of the test animals in relation to the mimic?
Nuclear physicists, years ago, had a similar problem. They wanted to observe the behaviour of their ‘fast’ and ‘slow’ particles. Wilson (1927) invented the cloud chamber, also called the Wilson chamber, to record the tracks of electrons. Glaser (1960) had to modify the technique to register the experimental behaviour of elementary particles. A Schlieren optical design gives the zooplankton experimenter similar capabilities (Strickler 1977). As mentioned above, the swimming animals deform the density gradient and the optics allow for visualizing the local density differences (Strickler & Hwang 1999; for additional techniques see Settles 2001).
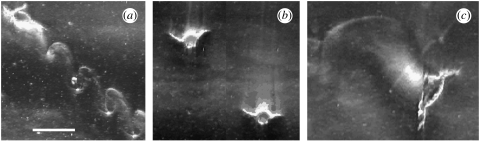
Let us analyse the basic footprints of cyclopoid and calanoid copepods. Cyclops scutifer swims with the typical hop-and-sink pattern (figure 2a; e.g. Alcaraz & Strickler 1988). However, when the animal receives fluid-dynamical signals, for example as it hops into a weak deformation of fluid generated by a neighbour animal, it stops swimming and sinks slowly (figure 2b). During this sinking phase, the antennules are fully stretched out to receive additional information. Conversely, during the short hop phase, the antennules are positioned alongside the body and may not be able to receive any information (Strickler & Bal 1973). Therefore, in the hop-and-sink pattern, receiving of information is interrupted by each hop.
Figure 2.
‘Footprints’ of C. scutifer and D. minutus. (a) Hop-and-sink swimming pattern of C. scutifer with one hop per second. (b) Two frames, 8 s apart, of the same C. scutifer sinking after it encountered a weak hydrodynamic disturbance. Note the small footprint left behind by the sinking animal. (c) Footprint created by a grazing D. minutus. Note the smooth outgoing feeding current leaving a less distinct pattern within the density gradient. Scale bar, 0.1 cm.
Many calanoid copepods create a feeding current. Diaptomus minutus, for example, swims backwards, advecting water and its entrained food particles from the layer above. This water, being from a lighter layer in the density gradient, will rise back to its layer (figure 2c). However, in nature, where there may not be a density gradient, the outflow of the feeding current is added to the layer below the animal (Jiang & Strickler 2007). In general, the animal generates the feeding current and moves very slowly backwards for approximately 30 s. Then, a small hop will reorient the animal within the water column. During this small hop, the animal may groom its mouthparts (Strickler 1984).
4. Reactions towards a mimic disturbance
We used a pipette containing the water from the upper-most layers of the density gradient in order to avoid any possibility that chemoreception could play a role. When an animal was within the field of view, a gentle release of a small volume of water from the pipette created a low Reynolds number jet and a deformation of fluid. Since the water was released from the pipette at some speed, the volume of water gained kinetic energy within the pipette, and while it moved down the gradient, its energy was used up. The small volume then moved up again close to the layer it came from due to buoyancy. The release of the water volume created a disturbance similar in size and motion to the one created by a fellow animal, so that we could expect to observe a natural reaction (Strickler 1975b).
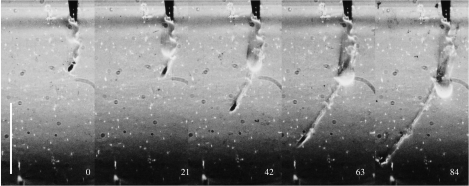
In figure 3, the animal, a female C. scutifer, was 0.3 cm from the nozzle of the pipette when the volume was released. In the following, we define time 0 as the frame where the leading edge of the water volume was visible at the nozzle of the pipette. Within 0.021 s, the animal initiated an escape reaction away from the disturbance, reaching a speed of 24 cm s−1 for 0.084 s and a distance of 1 cm. The jet left the nozzle with a speed of approximately 20 cm s−1.
Figure 3.
Cyclops scutifer escaping from an artificial hydrodynamic disturbance. For details see text. Numbers represent milliseconds, starting when the disturbance became visible. Scale bar, 1 cm.
When the pipette water was released at a much lower speed, 4.8 cm s−1, the reaction of the animal was less fierce (figure 4). It swam for a distance of 0.8 cm at a speed of approximately 5 cm s−1. The disturbance was created in front of the animal; however, at such low speed, the animal continued to swim in its original direction as did the animal in figure 3. With water released at the same speed, 4.8 cm s−1, but with the animal further away from the future path of the jet and oriented almost parallel to the jet, the animal waited for approximately 0.13 s before it induced an escape reaction (figure 5). The bulk of the released water was at the same level as the animal. The animal reoriented itself with one flip of the abdomen and with one power stroke of the antennules and swimming legs (see Strickler & Bal 1973 for details) and swam at 19 cm s−1 for approximately 1.2 cm. In figure 6, the animal reacted as soon as the jet started (Strickler 1975b). It swam at 18 cm s−1 for a distance of 2 cm, out of the view of the optics. The jet accelerated from 5 to 15 cm s−1 for the first 0.03 s after release and slowed down afterwards. Such an initial increase of mimic speed simulated even closer an attack by a predator and, therefore, the reaction was even more enhanced than with a solely decelerating jet.
Figure 4.
Cyclops scutifer escaping from a weak artificial hydrodynamic disturbance. Numbers represent milliseconds, starting when the disturbance became visible. Scale bar, 1 cm.
Figure 5.
Cyclops scutifer escaping from an off-track artificial hydrodynamic disturbance. The animal reacted after 0.13 s. Note the turn-about track when it reacted. Numbers represent milliseconds, starting when the disturbance became visible. Scale bar, 1 cm.
Figure 6.
‘Footprint’ of an escaping C. scutifer. The strong simulated disturbance triggered a fast escape with speeds up to 18 cm s−1; one power stroke for every 0.016 s (Strickler 1975b). Arrow points to the position of the animal when the disturbance was created. Scale bar, 1 cm.
Cyclops scutifer is a predator on smaller zooplankters, such as Bosmina spp., and rotifers. However, it is prey to the calanoid copepod Epischura nordenskioldi (Strickler & Twombly 1975). This animal swims for periods of up to 30 s with long swimming bouts at a constant speed, which are then interrupted with short reorientations. It does not create a feeding current and glides through the water with an average speed of 1.5 cm s−1. When it perceives a prey, it attacks it with a fast lunging motion before the prey can escape.
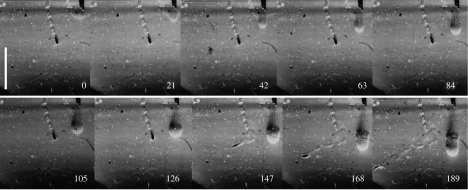
In figure 7, the jet had the same speed as in the experiment for figure 6. However, this time the test animal was a female E. nordenskioldi. The animal was approximately 1 cm away from the nozzle of the pipette when the water was released. For 0.021 s, the animal remained at the same spot. After 0.021 s, it initiated an attack. At 0.042 s, it swam at 20 cm s−1 towards the point where the bulk of the jet was at 0.021 s. At 0.063 s, it reached this spot and exerted a capture motion as seen by the disturbance created, and visualized at 0.084 s. At 0.084 s, the animal ‘realized’ that there was no prey to be captured and it left the spot at high speed. It then slowed down and unfolded its antennules at 0.126 s and resumed swimming and hunting at its cruising speed of 1.5 cm s−1.
Figure 7.
Epischura nordenskioldi attacking an artificial hydrodynamic disturbance. The disturbance resembled that which could be created by a hopping prey item. At 0.021 s, the animal determined the centre of the disturbance and, at 0.042 s, the animal attacked it at full speed, reaching this centre at 0.063 s. The animal then executed a capture motion with its mouthparts. This capture motion left a distinct ‘footprint’ visual at 0.084 s. The animal then continued swimming and at 0.126 s resumed a hunting position with stretched-out antennules. Numbers represent milliseconds, starting when the disturbance became visible. Scale bar, 1 cm.
5. Interaction between two conspecific animals
Escape reactions are energetically expensive (e.g. Strickler 1975a; Lenz & Hartline 1999; Buskey et al. 2002). When we conducted the experiments described above and tested the same animal several times, it did not react anymore. In figure 4, at the right edge of each frame, we can see two copepods, a lower whitish one and a darker one in the upper half of the frame, which do not react. Kils (1992) suggested on the basis of in situ observations that the herrings' tactics are to form schools to attack aggregates of copepods and tire out the copepods trying multiple times to escape capture. Here, we ask the question whether or not copepods will react every time they encounter a deformation of fluid signal which has been generated by a fellow zooplankter? Energetically, it would make sense if the animals did not react to signals generated by conspecific animals.
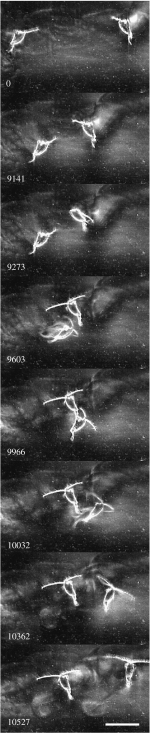
Approximately 20 female D. minutus (Calanoida: Copepoda) were observed swimming and feeding in 125 ml of water. Similar to the results of Strickler (1970), the animals avoided each other; only rarely they executed an escape reaction. Figure 8 shows one encounter between two animals. The feeding currents of these two animals started to intersect. To avoid interference, the two animals changed their directions (figure 8): the animal from the right at 9.273 s hopped to its right with one motion of its swimming legs and continued feeding at 9.603 s, and the animal from the left hopped to its right at 9.603 s and executed another hop at 10.032 s. It started to feed again at 10.362 s. Both animals continued to feed along these new tracks.
Figure 8.
Two grazing D. minutus on a collision course. For over 9 s, the distance between the two feeding animals diminished until their feeding currents drew water from the same position. The animal from the right initiated an avoidance manoeuvre first by executing one hop to its right. The animal from the left followed and executed two hops to its right. Numbers represent milliseconds, starting when both animals were within the volume of observation. Scale bar, 0.1 cm.
Jiang considered theoretically several geometries of two animals in close neighbourhoods of each other (Jiang et al. 2002). There were cases where togetherness was detrimental to the feeding rate. Folt experimented with calanoids and concluded that the larger species strongly influences the feeding rate of the smaller one (Folt & Goldman 1981). However, here one species could be the potential predator of the other and, therefore, additional changes in behaviour could be attributed to the presence of chemicals released by the animals.
6. Finding and following a female
From above, we conclude that the tested planktonic copepods display measured reactions towards small deformation-of-fluid signals. The change in behaviour, from escaping to reorienting when the information is generated by a conspecific animal, shows that the mechanoreceptors on the antennules, and probably on the other appendages like the antennae, allow the animals to receive spatially and temporally structured signals. This notion differs from the earlier assumption that copepods perform a vigorous escape reaction every time when perceiving a deformation of fluid of a strength that could have been generated by a zooplankter or fish. Strickler & Bal (1973) and Strickler (1975b) interpreted high-speed observations of encounters between C. scutifer. The conclusions were that hydrodynamic, not chemical, information elicits these escape reactions.
The question now arises of how a male finds its female and how he approaches her without inducing an escape reaction? To highlight this situation, we focused on an observation we described earlier (Strickler 1998). In that study, females and males of C. scutifer were caught from Lake Meach (Province de Quebec, Canada). For details on the recording procedure, please refer to the original publication (Strickler 1998). In this study, we analysed the data using more refined mathematical tools (Bendat & Piersol 2000). First, we defined the method of analysing time-series of each animal's position. And second, we tried to comprehend the mating tactics.
(a) The positions of the zooplankters
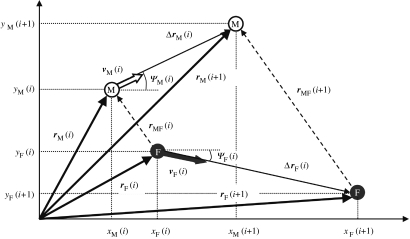
The vessel containing males and females and the camera system were mounted on an optical table and provided a fixed coordinate system. The observed sequence of events happened to be executed in a plane perpendicular to the optical axis allowing us to analyse the sequence in two dimensions, using the vertical and one horizontal coordinate only. The instantaneous position of male and female zooplankters (rM, rF) in the fixed coordinate system can be monitored by a sequence of measurements at equal time-intervals Δt. For example, the male zooplankter's position at the time of the ith measurement is given by (figure 9)
which gives the following identities for the Cartesian coordinates xM and yM:
Figure 9.
Illustration of the quantities used in the methods. The positions of male (white circle) and female (dark circle) at two consecutive time points (i and i+1) are shown. The origin of the coordinate system is the left lower corner of the video frames.
(b) The velocities of the zooplankters
The displacements ΔrM and ΔrF of zooplankters (figure 9) are the differences between their position vectors at two consecutive time points. The following expression approximates the male zooplankter's displacement at time ti+(Δt/2):
The velocity of zooplankters (vM and vF; figure 9) is defined as their displacement per unit time. For example, for the male zooplankter,
which implies the x- and y-components of the velocity
In addition, the velocities can be characterized by their magnitude (speed)
and the angle between their direction and the x-axis, ψM and ψF (figure 9),
(c) The relative positions of the zooplankters
In contrast to the position of zooplankters in a fixed coordinate system, their relative positions are determined by measuring the positions of the male zooplankter in a coordinate system attached to the female, with the x-axis aligned with the female's displacement. The relative position (figure 9) is defined as
and is characterized by its magnitude (or male–female distance) rMF,
with
and by the angle, φ, between the relative position vector and the fixed x-axis,
(d) The relative velocities of the zooplankters
The relative velocity of male and female zooplankters is defined as
The relative velocity is characterized by its magnitude (relative male–female speed) and the angle between the displacement of male and female. The change in male–female distance is defined as
Dividing the above equation by the time-step gives the relative male–female speed,
The angle between the male and female displacements is
(e) Coordination of male and female speeds
The coordination of male and female speeds, sM and sF, within a time window of size 2w+1, centred at i, ranging from i−w to i+w, can be quantified by using the coefficient of cross-correlation ρs(i),
where the mean male speed is
and the standard deviation of male speed is defined as
The coefficient of cross-correlation, ρs, takes values between −1 and 1, depending on the similarity between sM and sF within the time window (figure 10). For similar male and female speeds (male and female speed up and slow down simultaneously), ρs is positive. If the male and female speeds vary in opposite fashion (male speeds up when female slows down and vice versa), ρs is negative. Finally, when the male and female speeds are unrelated, ρs is close to 0.
Figure 10.
(a) The x-component of male–female distance. (b) The y-component of male–female distance. (c) The magnitude of male–female relative position vector (male–female distance). (d) The angle between male–female relative position vector and the x-axis. The alternating shading indicates periods 1–4.
(f) Coordination of male and female directions of movement
Similar to the correlation coefficient, the trigonometric cosine function takes values between −1 and 1. Moreover, when the male and female displacements are parallel, the cosine of their angle is 1. When the male and female displacements are opposite in direction, the cosine of their angle is −1. Finally, when the male and female displacements are perpendicular, the cosine of their angle is 0. Therefore, the cosine of the angle between male and female displacements, averaged over a time window, seems to be a suitable quantity to characterize (in addition to the coordination of speeds) the coordination of directions of movement between male and female. The average cosine between male and female displacement is defined as
(g) Coordination of male and female velocities
The velocity is a vector, characterized by its magnitude and direction. When studying the coordination of movement of male and female zooplankters, these two characteristics of the vector must be taken into account. One way to accomplish this is to calculate the cross-correlation of male and female velocities (instead of their speed), defined over a time window of size 2w+1, centred at i as
where the mean male velocity is
and the standard deviation of male velocity is defined as
7. Results: the relative positions of male and female C. scutifer
The relative positions of male and female C. scutifer, as well as their distances before, during and after mating, have been previously reported by Strickler (1998). However, the x- and y-components of the relative position rMF (figure 10a,b) and the cosine of the angle φMF between rMF and the x-axis (figure 10d) offer additional information on pre- and post-mating behaviour. For sake of completeness, we also show the male–female distance as a function of time (Strickler 1998) in figure 10c.
The character of the four graphs in figure 10 clearly changes with time, making it possible to distinguish four distinct periods. Initially, during period 1 (t<9 s), the magnitude of xMF, yMF and rMF have a decreasing tendency, indicating that the male gradually approached the female. During this period, cos(φMF) stayed near −1, exhibiting only small fluctuations. Over period 2 (9 s<t<30 s), xMF and rMF stayed at a constant low value, while yMF and cos(φMF) exhibited a very interesting fluctuation, repeatedly increasing and decreasing in time. The shortest of the four periods was period 3 (30 s<t<31 s), where xMF, yMF and rMF reached their lowest values, and cos(φMF) fluctuated rapidly, indicating rapid spinning of the animals. Finally, period 4 mirrored period 1, marked by a gradual increase in xMF, yMF and rMF, and small fluctuations of cos(φMF) around 1.
8. Results: the correlation coefficient and average cosine
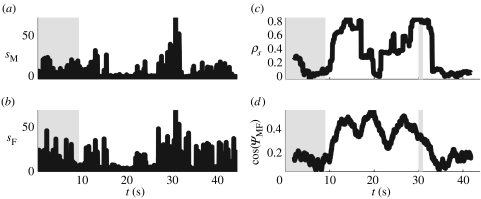
To characterize the similarity between male and female speeds (figure 11a,b), we calculated the cross-correlation coefficient ρs between the male and female speeds within a sliding time window of 4 s (100 frames). As figure 11c indicates, there was a substantial increase in the correlation coefficient when the sliding time window overlapped with periods 2 and 3. In addition, the average cosine of the angle between male and female displacement (figure 11d) was larger within the same periods. The correlation coefficient of velocities varied similarly to ρs over the four time periods of observation (results not shown). In addition, all of these quantities temporarily decreased immediately after t=20 s.
Figure 11.
(a) The speed of male C. scutifer. (b) The speed of female C. scutifer. (c) The cross-correlation coefficient of male and female speeds over a time window of 4 s (100 frames). (d) The average cosine of the angle between the speed of male and female over the time window. The alternating shading indicates periods 1–4.
9. Discussion: mating sequence
Investigating the mating behaviour of male and female C. scutifer, Strickler (1998) identified the following distinct periods of behaviour. During the first 10 s, neither male nor female perceives the presence of the other. During the next 20 s, the male follows the female, matching his temporal swimming pattern to hers jump by jump. We call this synchronization ‘tandem swimming’. During the following short period (less than 3 s), mating occurs. Finally, the male and female separate and continue their movements independently.
It is clear that the four periods uncovered by our methods in §8 are identical to the ones described before by Strickler (1998). However, our methods offer additional details of male and female behaviour during the four periods, which are distinguished from each other by (table 1): low speed and low correlation (periods 1 and 4), low speed and high correlation (period 2), and high speed and high correlation (period 3).
Table 1.
Individual male and female speeds (in mm s−1), relative male–female speed (in mm s−1) and the cosine of the angle between male and female displacements, averaged over time periods 1, 2, 3, and 4. (Means±s.d. are indicated.)
| SM (mm s−1) | SF (mm s−1) | SMF (mm s−1) | cos ψMF | |
|---|---|---|---|---|
| period 1: 0–9 s | 4.2714±4.6772 | 4.0065±6.3264 | 5.7553±6.9859 | 0.1339±0.7851 |
| period 2: 9–30 s | 2.9792±4.3580 | 3.6673±5.2168 | 3.5551±4.8273 | 0.3888±0.7004 |
| period 3: 30—31 s | 10.9790±14.7235 | 11.5800±15.5865 | 7.6552±7.0972 | 0.4118±0.5633 |
| period 4: 31–44 s | 3.3382±4.8723 | 4.7092±7.4492 | 5.7558±7.6063 | 0.1750±0.7082 |
In period 1, the distance between the male and female gradually decreased, and the male and female exhibited the hop-and-sink swimming behaviour characterized by jumps, as indicated by peaks in the male and female speed. One of the open questions regarding period 1 remains: whether the male and female arrive in each other's proximity as a result of random swimming. The continuously decreasing distance between them within the first 10 s seems to contradict this. One could assume that the male perceived some information about the presence of the nearby female and swam in the direction of the source of this information. However, since within this period the tracks of both animals are almost perpendicular to each other (see fig. 8 in Strickler 1998), we also would have to accept the notion that the male could predict the position of the female at the end of this 10 s period and swam directly to that position in order to intercept her; a notion not supported by any other observation of free-swimming copepods (e.g. Strickler 1970).
In period 2, the male used mechanoreception to perceive the female and the distance between them remained small and almost constant (figure 10c), indicating that the male successfully kept track of the female. In addition, the cosine of the angle φMF between their relative position vector and the x-axis remained negative (figure 10d), indicating that the male was always located on the same side of the female during the pre-mating pursuit. The repeated increase and decrease of this angle (figure 10d) and the y-coordinate (figure 10b) during period 2 probably indicates that the male attempted to remain at the female's outer edge of detection. The less frequent jumps of the female (figure 11b) and the generally decreased speed (figure 11b and table 1) might have served to increase the acuity of detection, provided that the female used hydrodynamic clues to ‘observe’ the male. During the stages of quiescence between jumps, the distance between the male and the female increased, but the male caught up with the female as soon as she started jumping again (the phases of repeatedly increasing and decreasing distance along the y-axis in figure 10b correspond to the quiescent stages and jumps). The female almost escaped around t=20 s, as indicated by the increasing distance rMF (figure 10c) and the dropping values of cross-correlation (figure 11c).
Ultimately, the pursuit was successful and mating occurred during a short period of time (period 3), when the male grabbed the female, minimizing the distance rMF between them (figure 10c). During this time, the angle φMF was not well defined (figure 10d), owing to the proximity and rapid spinning of male and female. The male and female speed increased dramatically during mating, indicated by the highest sM and sF (figure 11a,b). This rapid swimming is again suggestive of the female's escape attempts which were unsuccessful because the male grabbed her (the distance rMF is the lowest in this period).
After mating (period 4), the male and female continued their swimming, separately and independently. Many aspects of period 4 resemble period 1 (figure 10). The swimming pattern (frequency of jumps) returned to normal and the cross-correlation between the movements dropped to vanishingly small values (figure 11c,d). Once again, it is questionable whether the movement of male and female is completely random, at least within the temporal scales of our observations.
These results can be used to define thresholds for various types of behaviour. For example, from figure 10a, we can conclude that the male can perceive the female only if their distance rMF is less than 4 mm, noting that the male–female distance increased over this threshold around t=20 s when the female nearly escaped. The coincidence of the repeatedly increasing and decreasing values of yMF and φMF (figure 10b,d), with the jumps visible on the graphs of sM and sF (figure 11a,b), is suggestive of the nature of clues offered by the male and female during pursuit. These clues are hydrodynamic and are related to fast movement (jumps). The male observes the female's clues, but the female observes the approaching male as well as she jumps less frequently, attempting to ‘listen’ during the pursuit.
To conclude, the mathematical tools introduced here can be used to quantitatively identify mating behaviour in C. scutifer. These tools allow us to feasibly identify mating behaviour by only analysing recorded data, without actual observation of the animals. The methods should be tested on a larger number of individuals and, eventually, on different species. Our results allow deeper understanding of the details of mating behaviour, not evident to the observer (for example, the fact that the female nearly escaped around t=20 s would not have been obvious by simple observation of the animals).
10. Concluding remarks
Most of the observations and recordings on 16 mm film were made in the early 1970s. However, the tools were not available to comprehend the observed actions. We needed a clearer understanding of the water flow around a self-propelled planktonic copepod (Jiang & Osborn 2004). Use of models of towed entities did not explain what we have observed and the question remained about a possible information flow due to chemicals. Now, we are certain that the signals produced by the hop-and-sink swimming pattern are sufficient for mating. Since the sensory system is capable of guiding the male for 20 s to follow the female, it is also capable of distinguishing small disturbances from larger ones and ‘strange’ signals from conspecific ones.
There are still several open questions. Van Duren et al. (1998) observed that female calanoids, Temora longicornis, reacted to the odour produced by males. The females increased their activities in order to advertise their presence and increase the encounter probabilities with males. Bagoien & Kiorboe (2005) reported observations of mating in Acartia tonsa and analysed the data in terms of communication. However, whether or not we can talk of bidirectional communication between the mates is questionable. It would mean that the females were aware of the males and lead them to three-dimensional mating dances. But, in our observations, why then did the female try to escape when the male came very close to her? And, why did he stay for 20 s at the furthest distance possible, almost losing her? Further observations with different hop-and-sink planktonic animals may solve these questions.
Another question would be what is the adaptive value, if any, of tandem swimming? During the 20 s tandem swimming, the female swam with a rather random timing of hops and sinks. The male had to hop as soon as he received the signal that she induced a hop. Buskey & Hartline (2003) measured reaction times for hydrodynamic versus light stimuli in A. tonsa. The animals reacted within 0.003–0.006 s to the mechanical stimuli, well within the reaction time for the male to hop when the female hopped. In addition to mating, these animals need such fast reaction times to avoid predators, especially planktivorous fishes which capture copepods within 0.006 s (Coughlin & Strickler 1990).
Let us assume that females encounter males at random. How could the females select and mate with the best males? If females hop-and-sink with a strict frequency, for example one hop every second, males could key in to that frequency. However, and we have seen the same behaviour many times, our observation showed that the female displays a rather random temporal pattern of hops. The male has to key in to the signals received from the female and has to match her pattern as perfectly as possible, otherwise he would lose her due to an escape reaction. This means, the females' offspring will gain the genetic information to be adept in interpreting and reacting to hydrodynamical signals—an ever so important trait in escaping predators, saving energy while encountering conspecifics, and pursuing mates.
Tandem swimming could also serve to prevent interspecific crosses. Cyclopoid copepod species do not show much intraspecific variability in their morphologies (Huys & Boxshall 1991). Males and females use their swimming legs to hop (Alcaraz & Strickler 1988). Different sizes or shapes will produce different hops. Longer setae on the swimming legs, as for example a male from a different species would have, would result in a larger distance covered per hop, which in turn would shorten the distance between the male and the female, and would, for certain, trigger an escape reaction on her part. Similarly, shorter appendages create shorter hops and an increase in the distance; again, a losing proposition. Therefore, tandem swimming could function as an ethological isolation mechanism (Mayr 1963). However, further investigations are necessary because most harpacticoid copepods are also hop-and-sink swimmers and have very intriguing mating behaviours, including mate guarding and contact chemoreception (e.g. Kelly & Snell 1998; Palmer & Edmands 2000).
Acknowledgments
We thank Ai Nihongi and Dana Ono for the many hours they spent helping us in the laboratory, Sara Goetz for editing and two anonymous reviewers for their suggestions. J.R.S. acknowledges support from the National Science Foundation in grants to Ed Buskey (OCE 04-52159), Petra Lenz and Dan Hartline (OCE 04-51376) and J.R.S (OCE-0352264). G.B. acknowledges support from the National Science Foundation.
Footnotes
One contribution of 18 to a Theme Issue ‘Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms’.
References
- Alcaraz M, Strickler J.R. Locomotion in copepods: pattern of movements and energetics of Cyclops. Hydrobiologia. 1988;167/168:404–414. doi:10.1007/BF00026333 [Google Scholar]
- Bagoien E, Kiorboe T. Blind dating—mate finding in planktonic copepods. III. Hydromechanical communication in Acartia tonsa. Mar. Ecol. Prog. Ser. 2005;300:129–133. [Google Scholar]
- Bendat J.S, Piersol A.G. 3rd edn. Wiley-Interscience; New York, NY: 2000. Random data: analysis & measurement procedures. [Google Scholar]
- Buskey E.J, Hartline D.K. High-speed video analysis of the escape responses of the copepod Acartia tonsa to shadows. Biol. Bull. 2003;204:28–37. doi: 10.2307/1543493. doi:10.2307/1543493 [DOI] [PubMed] [Google Scholar]
- Buskey E.J, Lenz P.H, Hartline D.K. Escape behavior of planktonic copepods in response to hydrodynamic disturbances: high speed video analysis. Mar. Ecol. Prog. Ser. 2002;235:135–146. [Google Scholar]
- Claus C. Verlag Von Wilhelm Engelmann; Leipzig, Germany: 1863. Die frei-lebenden Copepoden. [Google Scholar]
- Costello J.H, Strickler J.R, Marrase C, Trager G, Zeller R, Freise A.J. Grazing in a turbulent environment: behavioral response of a calanoid copepod, Centropages hamatus. Proc. Natl Acad. Sci. USA. 1990;87:1648–1652. doi: 10.1073/pnas.87.5.1648. doi:10.1073/pnas.87.5.1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin D.J, Strickler J.R. Zooplankton capture by a coral reef fish: an adaptive response to evasive prey. Environ. Biol. Fish. 1990;29:35–42. doi:10.1007/BF00000566 [Google Scholar]
- Cuvier M. Deterville Libraire; Paris, France: 1829. Le règne animal. [Google Scholar]
- Folt C, Goldman C.R. Allelopathy between zooplankton: a mechanism for interference competition. Science. 1981;213:1133–1135. doi: 10.1126/science.213.4512.1133. doi:10.1126/science.213.4512.1133 [DOI] [PubMed] [Google Scholar]
- Glaser, D. A. 1960 Elementary particles and bubble chambers. In Nobel lectures in physics Singapore: World Scientific Publishing. See http://nobelprize.org/physics/laureates/1960/glaser-lecture.html
- Huys R, Boxshall G.A. The Ray Society; London, UK: 1991. Copepod evolution. [Google Scholar]
- Jiang H, Osborn T.R. Hydrodynamics of copepods: a review. Surv. Geophys. 2004;25:339–370. doi:10.1007/s10712-003-1282-6 [Google Scholar]
- Jiang H, Strickler J.R. Copepod flow modes and modulation: a modeling study of the water currents produced by an unsteadily swimming copepod. Phil. Trans. R. Soc. B. 2007;362:1959–1971. doi: 10.1098/rstb.2007.2081. doi:10.1098/rstb.2007.2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Osborn T.R, Meneveau C. Hydrodynamic interaction between two copepods: a numerical study. J. Plankton Res. 2002;24:235–253. doi:10.1093/plankt/24.3.235 [Google Scholar]
- Kelly L.S, Snell T.W. Role of surface glycoproteins in mate-guarding of the marine harpacticoid Tigriopus japonicus. Mar. Biol. 1998;130:605–612. doi:10.1007/s002270050282 [Google Scholar]
- Kerfoot W.C, Kellogg D.L, Strickler J.R. Visual observations of live zooplankters: evasion, escape, and chemical defenses. In: Kerfoot W.C, editor. Evolution and ecology of zooplankton communities. Special Symposium III American Society of Limnology and Oceanography. University Press of New England; Hanover, NH: 1980. pp. 10–27. [Google Scholar]
- Kils U. The EcoScope and dynImage: microscale tools for in situ studies of predator–prey interactions. Arch. Hydrobiol. Beih. 1992;36:83–96. [Google Scholar]
- Lenz P.H, Hartline D.K. Reaction times and force production during escape behavior of a calanoid copepod, Undinula vulgaris. Mar. Biol. 1999;133:249–258. doi:10.1007/s002270050464 [Google Scholar]
- Mayr E. Belknap Press; Cambridge, MA: 1963. Animal species and evolution. [Google Scholar]
- Palmer C.A, Edmands S. Mate choice in the face of both inbreeding and outbreeding depression in the intertidal copepod Tigriopus californicus. Mar. Biol. 2000;136:693–698. doi:10.1007/s002270050729 [Google Scholar]
- Settles G.S. Springer; Berlin, Germany: 2001. Schlieren and shadowgraph techniques. [Google Scholar]
- Strickler J.R. Über das Schwimmverhalten von Cyclopoiden bei Verminderungen der Betrahlungsstärke. Schweiz. Z. Hydrol. 1970;32:150–180. doi:10.1007/BF02502402 [Google Scholar]
- Strickler J.R. Swimming of planktonic Cyclops species (Copepoda, Crustacea): pattern, movements and their control. In: Wu T.Y.-T, Brokaw C.J, Brennan C, editors. Swimming and flying in nature. Plenum Press; New York, NY: 1975a. pp. 599–613. [Google Scholar]
- Strickler J.R. Intra- and interspecific information flow among planktonic copepods: receptors. Int. Ver. Theor. Angew. Limnol. Verh. 1975b;19:2951–2958. [Google Scholar]
- Strickler J.R. Observation of swimming performances of planktonic copepods. Limnol. Oceanogr. 1977;22:165–170. [Google Scholar]
- Strickler J.R. Sticky water: a selective force in copepod evolution. In: Meyers D.G, Strickler J.R, editors. Trophic interactions within aquatic ecosystems. Westview Press; Boulder, CO: 1984. pp. 187–239. [Google Scholar]
- Strickler J.R. Observing free-swimming copepods mating. Phil. Trans. R. Soc. B. 1998;353:671–680. doi:10.1098/rstb.1998.0233 [Google Scholar]
- Strickler J.R, Bal A.K. Setae of the first antennae of the copepod Cyclops scutifer (Sars): their structure and importance. Proc. Natl Acad. Sci. USA. 1973;70:2656–2659. doi: 10.1073/pnas.70.9.2656. doi:10.1073/pnas.70.9.2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler J.R, Hwang J.-S. Matched spatial filters in long working distance microscopy of phase objects. In: Cheng P.C, Hwang P.P, Wu J.L, Wang G, Kim H, editors. Focus on multidimensional microscopy. World Scientific Publishing; River Edge, NJ: 1999. pp. 217–239. [Google Scholar]
- Strickler J.R, Twombly S. Reynolds number, diapause and predatory copepods. Int. Ver. Theor. Angew. Limnol. Verh. 1975;19:2943–2950. [Google Scholar]
- Toepler A. Über die Methode der Schlierenbeobachtung als mikroskopisches Hilfsmittel, nebst Bemerkungen zur Theorie der schiefen Beleuchtung. Poggendorf's Ann. Phys. Chem. 1866;127:556–580. [Google Scholar]
- Van Duren L.A, Stamhuis E.J, Videler J.J. Reading the copepod personal ads: increasing encounter probability with hydromechanical signals. Phil. Trans. R. Soc. B. 1998;353:691–700. doi:10.1098/rstb.1998.0235 [Google Scholar]
- Vogel S. Princeton University Press; Princeton, NJ: 2003. Comparative biomechanics: life's physical world. [Google Scholar]
- Wilson, C. T. R. 1927 On the cloud method of making visible ions and the tracks of ionizing particles. In Nobel lectures in physics Singapore: World Scientific Publishing. See http://nobelprize.org/physics/laureates/1927/wilson-lecture.html
- Yen J, Strickler J.R. Advertisement and concealment in the plankton: what makes a copepod hydrodynamically conspicuous? Invert. Biol. 1996;115:191–205. doi:10.2307/3226930 [Google Scholar]