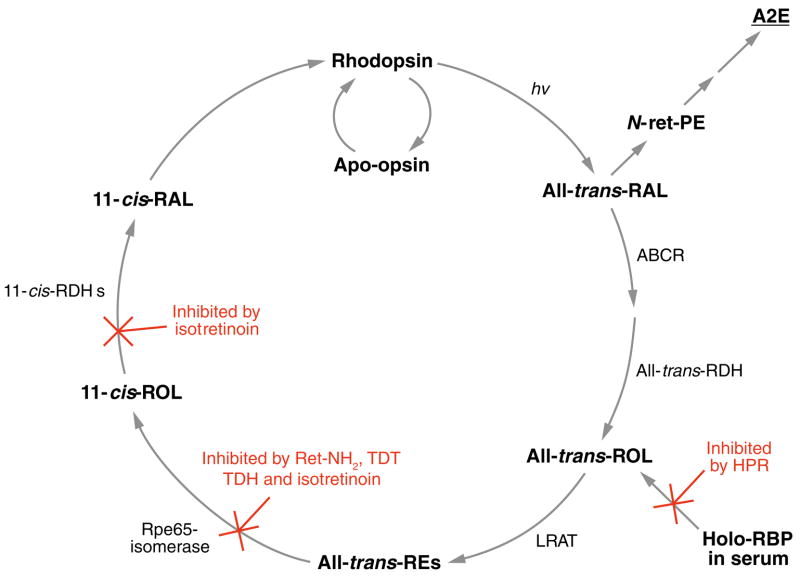
Figure 5.
Simplified visual cycle showing sites of action for the inhibitors of N-retinylidene-N-retinyl ethanolamine (A2E) formation. All-trans-RAL released by photoactivated rhodopsin condenses with phosphatidylethanolamine (PE) in disc membranes to form N-retinylidene-PE (N-ret-PE). Secondary condensation of N-ret-PE with another all-trans-RAL, followed by electrocyclization, hydrolysis of the phosphate ester, and oxidation yields A2E. Pharmacologic interventions that reduce light-dependent formation of all-trans-RAL greatly reduce the probability of this secondary condensation and inhibit formation of A2E. N-(4-hydroxyphenyl)retinamide (HPR) acts to reduce levels of holo-retinol binding protein (RBP) in serum. Rpe65-mediated isomerization can be blocked by retinylamine (Ret-NH2), the farnesyl-containing analogues, TDH and TDT, and isotretinoin. Isotretinoin also inhibits 11-cis-ROL dehydrogenase (11-cis-RDH).