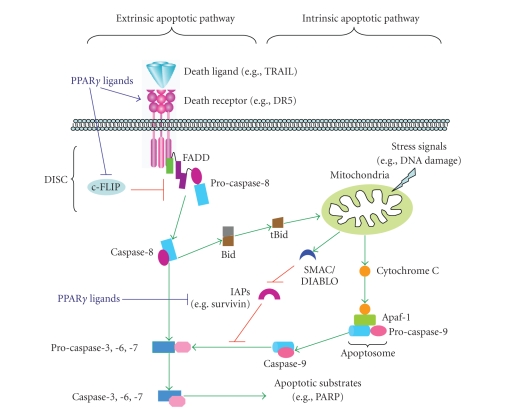
Figure 1.
Schema for basic apoptotic signaling pathways and possible mechanisms underlying PPARγ ligand-induced apoptosis. Ligation of death ligands (e.g., TRAIL) with their receptors (e.g., DR5) results in formation of the death-inducing signaling complex (DISC), in which pro-caspase-8 will be recruited through the death adaptor protein FADD and cleaved to generate activated caspase-8. This process is inhibited by c-FLIP. Certain stress signals (e.g., DNA damage) can target mitochondria and induce cytochrome C release from the mitochondria into the cytosol leading to caspase-9 activation by forming an apoptosome via binding to Apaf-1. Both caspase-8 and caspase-9 activate downstream procaspase-3, -6, and -7, leading to cleavages of their target death proteins such as PARP. In addition, truncated Bid (tBid), activated by caspase-8 via cleavage, facilitates insertion of Bax into the mitochondrial membrane leading to cytochrome C release. Therefore, tBid may serve as a link between the extrinsic and intrinsic apoptotic pathways. Inhibitors of apoptosis proteins (IAPs) such as survivin can bind to activated caspase-9 and prevent its action on effector caspases, whereas SMAC/DIABLO binds to IAPs, leaving caspase-9 free to activate the effector caspases. PPARγ ligands may induce apoptosis through induction of DR5 and/or downregulation of c-FLIP and/or survivin.