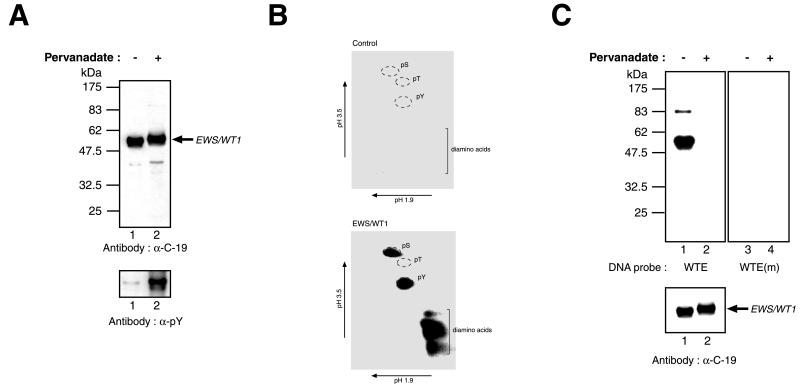
Figure 1.
In vivo phosphorylation of EWS/WT1. (A) EWS/WT1 is a phosphotyrosine protein. Cells transfected with pcDNA3.1-(His)6EWS/WT1(−KTS) were treated with vehicle (−) or pervanadate (+) 15 min before harvesting. Western blots were performed on Ni2+-affinity-purified protein preparations prepared from cell lysates and resolved on 10% SDS-polyacrylamide gels. The nature of the antibody used in the Western blot is indicated below each panel. The arrow indicates the position of migration of the EWS/WT1 product. Molecular mass markers are provided to the left and are derived from prestained New England Biolabs (NEB) protein standards (broad range). (B) Phosphoamino acid analysis of (His)6-EWS/WT1. Transiently transfected COS cells were metabolically labeled with 0.85 mCi/ml inorganic pyrophosphate (NEN) in phosphate-free media supplemented with 10% dialyzed FBS for 4 hr. Pervanadate solution was added directly to the labeling media for 15 min, and cells were harvested and lysed by sonication in RIPA buffer (50 mM Tris⋅HCl, pH 8.0/150 mM NaCl/1.0% NP-40/0.5% sodium deoxycholate/0.1% SDS). After preclearing of extracts with rabbit preimmune serum and protein G Sepharose, immunoprecipitations were performed with the C-19 antibody (Santa Cruz Biotechnology). Bound protein was eluted under denaturing conditions (8 M urea, 0.1 M sodium phosphate, 0.01 M Tris⋅HCl, pH 8.0), applied to Ni-nitrilotriacetic acid agarose resin (Qiagen), washed twice in wash I (8 M urea, 0.1 M sodium phosphate, 0.1 M Tris⋅HCl, pH 6.3), four times in wash II (40 mM sodium phosphate, pH 8.0, 300 mM NaCl, 30 mM immidazole), eluted with elution buffer (8 M urea/0.1 M sodium phosphate/0.01 M Tris⋅HCl, pH 6.3/100 mM EDTA), and separated on a 10% SDS/PAGE. Proteins were transferred onto Immobilon PVDF membrane and (His)6-EWS/WT1-identified by autoradiography. The same molecular mass region was excised from the PVDF membrane, which contained extract prepared from control cells transfected with empty vector. Hydrolysis was performed in 6 M HCl at 110°C for 1 hr, after which time 1 ml of water was added and samples were lyophilized. Samples then were resuspended in 10 μl electrophoresis buffer (0.58 M formic acid/1.36 M acetic acid, pH 1.9), spotted onto cellulose thin-layer plates, and analyzed by two-dimensional electrophoresis at pH 1.9 (0.58 M formic acid, 1.36 M acetic acid) and at pH 3.5 [0.87 M acetic acid, 0.5% (vol/vol) pyridine, 0.5 mM EDTA]. Nonradioactive standards were visualized by staining with ninhydrin in acetone and labeled amino acids were detected by autoradiography. (C) Southwestern blot analysis was performed on EWS/WT1(−KTS) isolated from transfected COS-7 cells treated with only vehicle (−) or with pervanadate (+) and fractionated on a 10% SDS/PAGE (Upper). After guanidine HCl denaturation/renaturation washes, the blot was probed with a 32P end-labeled oligonucleotide containing the wild-type WT1 recognition sequence (WTE) or a mutant WT1 motif [WTE(m)]. After washes, the blots were exposed to x-ray film (Kodak) at −70°C overnight with an intensifying screen. Immunoblotting with an anti-EWS/WT1 antibody (α-C-19, Santa Cruz Biotechnology) was used to confirm that equivalent levels of protein were loaded on the gels (Lower). The arrow indicates the position of migration of EWS/WT1. Molecular mass markers are provided to the left and are derived from prestained NEB protein standards (broad range).