Figure 4.
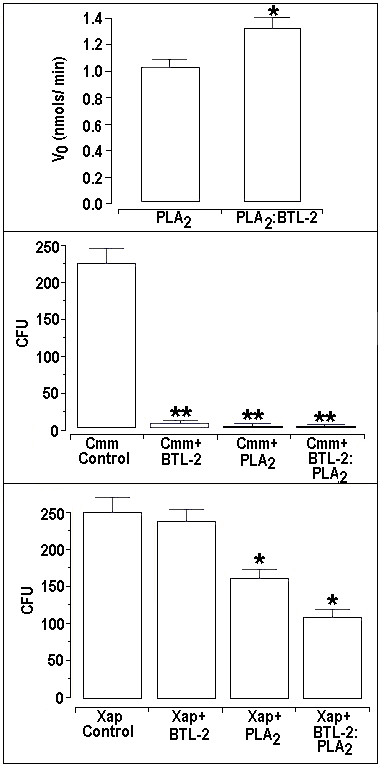
4a) The enzymatic activity assay [45] was analyzed using an Elisa plate containing 200 μL of buffer (10 mM Tris-HCl, 10 mM CaCl2, 100 mM NaCl, pH 8.0), 20 μL of substrate (3 mM, 4-nitro-3-octanoyloxy-bencoic acid), 20 μL of water and 20 μL of the sample at a concentration 1 μg/μL or 2 μg/μL when both proteins were incubated together. After 20 minutes from the beginning of the reaction, the absorbance was measured at 425 nm using a SpectraMax 340 multiwell plate reader (Molecular Devices). It is possible to observe that the enzymatic activity of the PLA2 was increased in the presence of BTL-2. 4b) and 4c) Clavibacter michiganensis subsp. michiganensis and Xanthomonas axonopodis pv.passiflorae were harvested from fresh agar plates and suspended in distilled and sterilized water (A600 nm = absorbance). BTL-2 demonstrated an inhibition of 98% of the growth of the Gram-positive bacterial strain, Clavibacter michiganensis subsp. michiganensis (Cmm), but only a 9.8% inhibition of the Gram-negative bacterial strain, Xanthomonas axonopodis pv passiflorae (Xap). PLA2 decreased Xap bacterial growth by 28.3% and Cmm growth by 98.5%, whilst incubation of these two proteins PLA2-BTL-2 inhibited their growths by 36.2% for Xap and 98.5% for Cmm.
