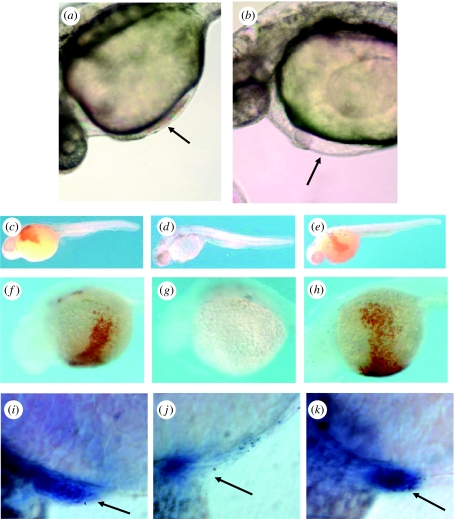
Figure 6.
Suppression of red blood cell formation in zebrafish embryos by antisense MOs targeted to the bty mRNA. (a,b) Differential interference contrast microscopy (DIC) of zebrafish embryos in vivo, 28 hpf (Nikon Eclipse 800 microscope). (a) Non-injected, wild-type embryo. The arrow shows circulating erythrocytes in the heart. (b) Wild-type embryo injected at the two cell stage with 10 ng of an antisense MO-1 targeted to the bty start codon and 21 bases downstream. Although the heart beats normally, circulating blood cells were absent (arrow). (c–e) Haemoglobin detection by o-dianisidine, whole embryo lateral view (32 hpf). Fixed and stained embryos were micrographed in 70% glycerol/PBS using a Nikon dissecting microscope. (c) Control wild-type embryo injected with MO buffer only. The circulation stained reddish brown when reacted with o-dianisidine, indicating the presence of haemoglobin-expressing red cells. (d) Antisense MO-2 directed to the 5′-UTR of the bty mRNA, 5 ng. Note the nearly complete absence of red blood cells. (e) MO-2 control with 5-bp mismatch, 5 ng. Red blood cells were present at wild-type levels. (f–h) Haemoglobin detection by o-dianisidine, view of venous circulation over yolk to heart. Embryos were micrographed as in (c–e). (f) Uninjected wild-type embryo. (g) Antisense MO-2, 10 ng. (h) Control 4-bp mismatch MO, 10 ng. (i–k) Whole-mount in situ hybridization of MO-treated zebrafish to α-globin antisense RNA (32 hpf). Micrographs were recorded in DIC mode (see a,b). (i) Uninjected embryo. (j) Embryo injected with 10 ng of antisense MO-1. (k) Embryo injected with 15 ng of control 4-bp mismatch MO. The arrows indicate the position of the heart. Globin-mRNA-positive red cells were greatly reduced in the experimental MO-injected embryo. Reprinted with permission from Yergeau et al. (2005). Copyright © 2005 Elsevier, Inc.