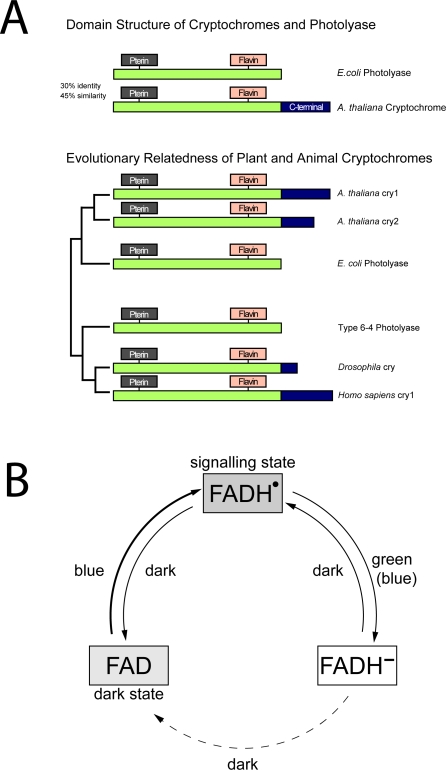
Figure 1. Cryptochromes and DNA Photolyases.
(A) Evolutionary relatedness and domain structure of plant and animal cryptochromes. N-terminal region is highly conserved and binds the same light-absorbing cofactors: a folate at the N-terminal region and a catalytic flavin chromophore at the C-terminal region. Plant cryptochromes are most related to Type I CPD photolyases, whereas animal cryptochromes are most related to 6–4 photolyases.
(B) The photocycle of plant cryptochromes. In the dark, the flavin chromophore is in its oxidized redox state. Blue light induces conversion to a meta-stable semiquinone redox state that is the activated signaling state. Green light causes further reduction to the fully reduced redox state of flavin, which is inactive in signaling. In the dark, fully reduced flavin reoxidizes to the fully oxidized form and can be reactivated by blue light. The photocycle of plant cryptochromes is different from DNA photolyases, in which only the fully reduced redox state is catalytically active.