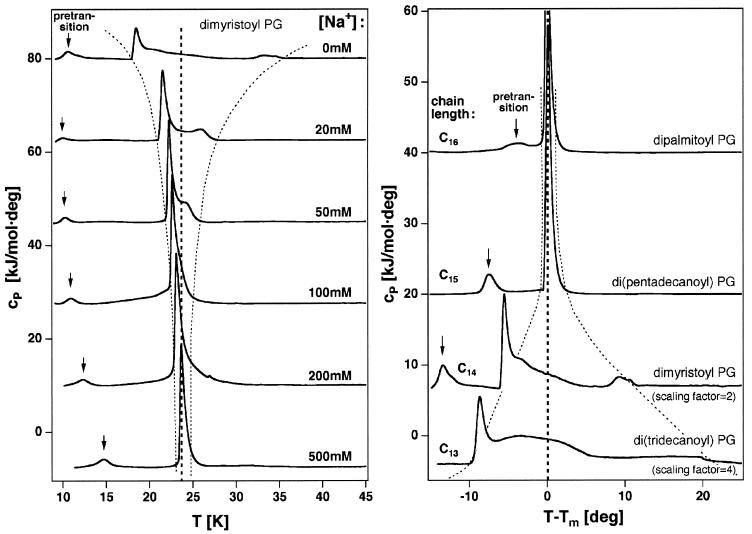
Figure 3.
(Left) Heat capacity traces of a 10 mM DMPG dispersion under various ionic strength conditions (in a 2 mM Hepes, 1 mM EDTA, pH 7.5 buffer; the top trace was measured in distilled water). At low ionic strength the Cp traces show a complex behavior as in Fig. 1. With increasing NaCl concentration the chain of events occurs over a narrower temperature interval. At 500 mM Na+ one single, highly cooperative heat capacity peak is found. Below the main transition the pretransition can be found (indicated by an arrow). This low enthalpy transition is not considered here in detail. It is, however, also linked to minor structural changes (formation of ripples on the surface, visible on the membrane surface in Fig. 2B Left. (Right) Heat capacity traces of 10 mM dispersions of phosphatidylglycerols with various chain lengths (in distilled water, pH ≈7.5). Di(tridecanoyl) PG (C13 chains) and dimyristoyl PG (C14 chains) show a complex behavior as in Fig. 1. With increasing chain length the chain of events occurs over a narrower temperature interval. Dipalmitoyl PG (C16 chains) displays only one very cooperative heat capacity peak.