Abstract
How is behaviour organised across sensory modalities? Specifically, we ask concerning the fruit fly Drosophila melanogaster how visual context affects olfactory learning and recall and whether information about visual context is getting integrated into olfactory memory. We find that changing visual context between training and test does not deteriorate olfactory memory scores, suggesting that these olfactory memories can drive behaviour despite a mismatch of visual context between training and test. Rather, both the establishment and the recall of olfactory memory are generally facilitated by light. In a follow-up experiment, we find no evidence for learning about combinations of odours and visual context as predictors for reinforcement even after explicit training in a so-called biconditional discrimination task. Thus, a ‘true’ interaction between visual and olfactory modalities is not evident; instead, light seems to influence olfactory learning and recall unspecifically, for example by altering motor activity, alertness or olfactory acuity.
Keywords: Olfaction, Vision, Learning, Context, Biconditional discrimination
Introduction
Animals need to simultaneously deal with stimuli from different sensory modalities. Choosing which to ignore, respond to or learn about is thus a biologically important task, potentially requiring a cross-talk between sensory modalities and an integration with the particular behavioural demands. Whether such cross-talk can be demonstrated in insect behaviour and how the relatively simple brains of insects may accomplish such tasks are thus interesting questions for basic research (examples of such analyses of cross-talk between sensory modalities come from various species: fruit fly: Guo and Guo 2005; honeybee: Gerber and Smith 1998; cricket: Matsumoto and Mizunami 2004; cockroach: Sato et al. 2006; bumblebee: Fauria et al. 2002; for examples concerning non-insect invertebrates: Hvorecny et al. 2007; see “Discussion” for details).
We use the fruit fly Drosophila melanogaster to explore interactions between olfactory and visual modalities. Fruit flies readily learn odours as predictors for an aversive electric shock (Tully and Quinn 1985). In addition, flies can associate illumination, color or patterns with reinforcement (Heisenberg 1989; Wolf and Heisenberg 1991). Olfactory (reviewed in Gerber et al. 2004), and to some extent also visual learning (Liu et al. 2006), in fruit flies are fairly well studied at the cellular and molecular level, but the interaction between them has so far not been investigated. The present study asks how visual context affects olfactory learning and recall and whether fruit flies integrate the information about the visual context into their olfactory memory.
Materials and methods
Flies and experimental setup
D. melanogaster of the Canton-Special wild-type strain are maintained as mass culture at 25°C, 60–70% relative humidity and under a 14:10-h light/dark cycle. On the day before experiments, 1- to 4-day-old flies are collected in fresh food vials and kept overnight at 18°C and 60–70% relative humidity. Experiments are performed at 22–25°C and 75–85% relative humidity, either under dim red light which does not allow flies to see (dark) or with illumination from a 50-W light bulb, placed ∼50 cm above the experimental setup (light). Flies are trained and tested in groups of ∼100. As odourants, 90 μl benzaldehyde (BA) or 340 μl 3-octanol (OCT; both from Fluka, Steinheim, Germany) are applied undiluted in 1-cm-deep Teflon containers of 5- and 14-mm diameters, respectively. Otherwise, the setup is as described by Schwaerzel et al. (2003).
Effect of visual context on olfactory learning and recall
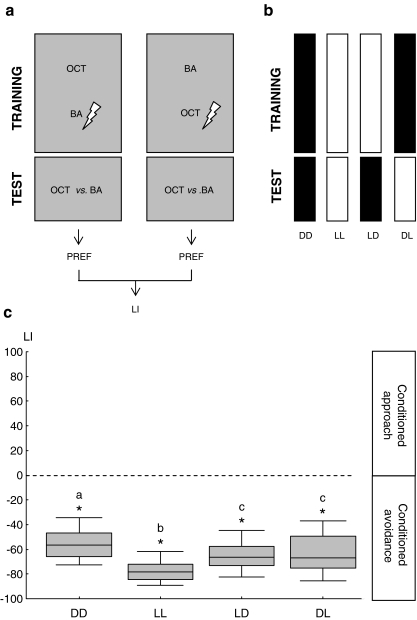
We compare levels of olfactory learning and recall under four conditions (Fig. 1b): training and test in dark (DD); training and test in light (LL); training in light, test in dark (LD); training in dark, test in light (DL).
Fig. 1.
Light facilitates both establishment and recall of olfactory memory. a For each visual condition, we train two groups: one receives 3-octanol (OCT) as the control odour, while benzaldehyde (BA) is paired with electric shock, whereas the second group is trained reciprocally. Each group is then given the choice between the two odours. Associative learning results in avoidance of the previously punished odour. We calculate a learning index (LI) based on the difference between odour preferences (PREF) of the two reciprocally trained groups. Negative LIs indicate avoidance of the learned odour. b Based on the reciprocal design detailed in a, flies are either trained and tested in dark (DD); trained and tested in light (LL); trained in light, tested in dark (LD) or trained in dark tested in light (DL). c In all groups, significant learning scores are found. Comparing between groups, flies perform poorest in DD and best in LL. The LD and DL conditions support intermediate performance
Training starts by loading the flies into the setup (0:00). The control odour is presented from 4:00 min to 5:00 min. The to-be-learned odour follows from 6:00 min to 7:00 min. At 6:15 min, electric shock is given as 12 pulses of 100 V, each 1.2 s long, with 5 s inter-pulse interval. At 13:00 min, flies are transferred for testing to the choice point of a T-maze where they can distribute between the two odours for 2 min. These parameters follow Schwaerzel et al. (2003). In half of the cases, we use BA as the to-be-learned and OCT as the control odour (OCT/ BA-Shock), whereas in the other half of the cases, training is reciprocal (BA/ OCT-Shock; Fig. 1a). In half of the cases, training starts with the control odour, whereas in the other half, the to-be-learned odour has precedence. We determine the number of flies in each arm of the maze and calculate an odour preference (PREF) as:
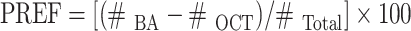 |
1 |
A learning index (LI) is then calculated as the difference in preference between the reciprocally trained groups:
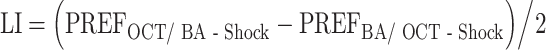 |
2 |
PREFOCT/ BA-Shock and PREFBA/ OCT-Shock denote the preferences of the respectively trained groups. Negative LIs indicate avoidance of the learned odour.
We use one-sample sign tests to compare the LIs of each group to zero. A Bonferroni correction keeps the experiment-wide error rate at 5% by dividing the significance level α by the number of comparisons (e.g. in the case with four comparisons α = 0.05/4). For comparing LIs between groups, we use a 2 × 2 factorial analysis of variance (ANOVA) after having probed for normality by the Lilliefors test. We present the data as box plots; in these plots, the midline represents the median, whereas box-boundaries and whiskers represent the 25% and 75% as well as 10% and 90% quartiles, respectively.
Biconditional discrimination
To test whether with explicit training Drosophila can establish visual context-dependent olfactory memories, we use a so-called ‘biconditional discrimination’ design. For a given group of flies, one odour is paired with shock in light, but not in darkness; another odour, in turn, is paired with shock in darkness, but not in light. Thus, neither the odours nor the visual situation alone can unambiguously predict shock—only the combinations of both can.
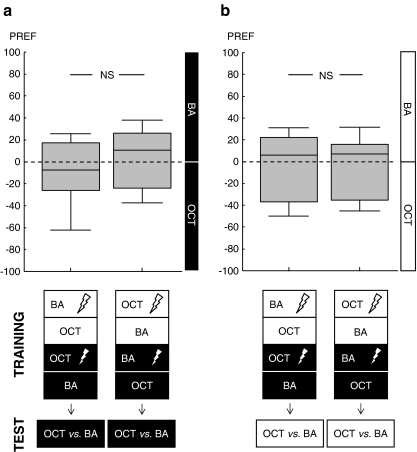
We use four groups (Fig. 2a,b): one group, in light, receives shock with BA, but not with OCT, whereas in darkness, contingencies are reversed. A second group is trained reciprocally. Both groups are then tested in dark for their preference between BA and OCT. The two further groups are trained the same as the ones already mentioned, but are tested in light.
Fig. 2.
No evidence for biconditional discrimination. a The first group of flies is trained such that in light, benzaldehyde (BA) is punished but 3-octanol (OCT) is not, whereas in dark, contingencies are reversed. The second group is trained reciprocally. When tested in dark, the first group should avoid OCT more strongly relative to the second group, which is not the case. b The third group of flies is trained such that in light, BA is punished but OCT is not, whereas in darkness, contingencies are reversed. The fourth group is trained reciprocally. When tested in light, the third group should avoid BA more strongly relative to the fourth group, which is not the case. c, d Flies are trained and tested in darkness with one of the odours unambiguously paired with shock, otherwise keeping the parameters of training and test as in a and b. c After punishment of BA, flies avoid BA stronger relative to the reciprocally trained group. d Learning indices calculated from these odour preferences are significantly different from zero, arguing that learning and recall are possible under these conditions
Reasoning that biconditional discrimination is a more difficult task for the flies to master and that it may require some repetition, we use more but ‘weaker’ training trials than in the first experiment: Training consists of six blocks, each with the four respective kinds of training trial. Across repetitions of the experiment, we pseudo-randomise the order of trials, avoiding two ‘shocked-trials’ in a row. Each trial lasts 2 min and is immediately followed by the next. At 0:00 min, visual context is set. Odour is presented at 0:45 min for 15 s. In ‘shocked-trials’, shock is presented at 1:00 min as four pulses of 100 V, each 1.2 s long and with 5 s inter-pulse interval. Thus, odour precedes shock with an onset-to-onset interval of 15 s; the visual context (either light or dark), on the other hand, spans the entire training trial. At 5 min after the end of the last training trial, flies are transferred to the choice point of a T-maze and are allowed 2 min to distribute between the two odours. The visual context during this 2-min choice period then can be light or dark. The PREF values are calculated according to Eq. 1. We compare the PREF values between reciprocally trained groups with a Mann–Whitney U test.
In a follow-up experiment, we test for ‘usual’ odour-shock learning and recall using the same training and test parameters as in the biconditional discrimination experiment; ‘usual’ here means that the complete experiment is run in darkness and by reliably pairing one odour with shock (Fig. 2c). Specifically, we use two reciprocally trained groups (i.e. OCT/ BA-Shock and BA/ OCT-Shock), whose PREF values are calculated according to Eq. 1 for comparison to each other with a Mann–Whitney U test. Based on the difference in preference between reciprocally trained groups, we additionally calculate LIs as in Eq. 2. LIs are compared to zero using a one-sample sign test.
Results
Light facilitates both establishment and recall of olfactory memory
We compare the level of olfactory learning and recall under four conditions (Fig. 1b): training and test in dark (DD); training and test in light (LL); training in light, test in dark (LD); training in dark, test in light (DL). We find significant learning scores for each of the four conditions (Fig. 1c; one-sample sign tests: for each condition: α = 0.05/4; P < 0.001; sample sizes n = 33, 33, 30, 34). Comparing between conditions reveals that flies show the poorest scores when training and test happen in dark (DD) and do best when both happen in light (LL). When only training (LD) or only test (DL) happen in light, performance is intermediate [Fig. 1c; 2 × 2 factorial ANOVA: effect of training context: α = 0.05, F1,126 = 16.68, P < 0.001; effect of test context: α = 0.05, F1,126 = 14.39, P < 0.001; interaction of effects: α = 0.05, F1,126 = 0.81, P = 0.37; each condition gives normally distributed LIs (Lilliefors test α = 0.05; P > 0.2, each) fulfilling the prerequisite for an ANOVA; sample sizes as above]. Thus, both olfactory learning and recall are generally enhanced by light. As it does not matter whether the visual context matches between training and test (see lack of significant interaction above), information about the visual context does not seem to be integrated into olfactory memory. Importantly, the kind of training used in this experiment allows flies to predict shock based on odours alone; in the following experiment, in contrast, we demand flies to learn about the visual context as well as about the odours.
No evidence for biconditional discrimination across visual and olfactory modalities
We run a ‘biconditional discrimination’ experiment where one odour is paired with shock in light but not in darkness; another odour, in turn, is paired with shock in darkness, but not in light (see sketches in Fig. 2a, b). Thus, neither the odours nor the visual context can unequivocally predict shock; only if flies were able to consider the combinations of both could they avoid danger. Biconditional discrimination should result in a difference in odour preference between reciprocally trained groups. However, neither when being tested in dark nor when tested in light do the reciprocally trained groups differ in their behaviour (test in dark: Fig. 2a, Mann–Whitney U test: α = 0.05, U = 94.00, P = 0.32, sample sizes n = 15, 16; test in light: Fig. 2b, Mann–Whitney U test: α = 0.05, U = 107.00, P = 0.84, sample sizes n = 15, 15). This lack of effect does not appear to be due to low statistical power because testing in light reveals no evidence for a difference between the reciprocally trained groups (the P value equals 0.84), and for testing in dark, if anything, we observe a tendency in the ‘wrong direction’. Thus, we find no evidence for biconditional discrimination.
Could this reflect adverse effects of the high number of shock-pulses during training (48 instead of 12; e.g. Schwaerzel et al. 2003) or the long duration of training (∼50 min instead of ∼10 min)? We run a ‘normal’ odour-shock learning experiment (i.e. training and test are performed in darkness; one odour is reliably paired with shock), otherwise using the same training and test parameters as in the previous experiment. We find that learning and recall are possible under these conditions: The two reciprocally trained groups differ in their odour preference (Fig. 2c; Mann–Whitney U test: α = 0.05, U = 0.00, P = 0.002, sample sizes n = 6, 6), resulting in significant learning scores calculated based on this difference in preference (Fig. 2d; one-sample sign test: α = 0.05, P = 0.03, sample size n = 6). Thus, as far as the olfactory modality is concerned, the training and test parameters of the biconditional discrimination experiment are in principle adequate. As for the visual modality, although we do not explicitly test for the learning of light versus dark, the result of the previous experiment (Fig. 1) argues that these two contexts sufficiently differ from each other to matter for the flies’ behaviour.
We conclude from our experiments that there is no evidence for across-modality biconditional discrimination; clearly, absence of proof is not proof of absence. However, in principle, our experimental design seems appropriate. First, successful biconditional discrimination in crickets (Matsumoto and Mizunami 2004) and cockroaches (Sato et al. 2006) also used ‘light’ versus ‘dark’ as visual contexts. Second, the number of trials for biconditional discrimination training was chosen to match the number of trials required for asymptotic elemental learning, both in our case (Fig. 2d) and in crickets (Matsumoto and Mizunami 2000, 2004). Finally, all three experimental designs involve training with four combinations of olfactory and visual stimuli, but use only two of them at test. Testing with all four combinations, at least in larval fruit flies, does not reveal biconditional discrimination, either (Yarali et al. 2006).
Discussion
We find no evidence for biconditional discrimination using combinations of visual and olfactory cues in fruit flies, speaking against interaction between the two modalities; however, both the establishment and the recall of olfactory memory are facilitated by light, apparently speaking in favour of such interaction. How can these findings be reconciled?
We structure this discussion considering the possible site of interaction along the sensory-motor continuum. That is, processing of different sensory modalities may interact ‘truly’ in the sense that the interaction is stimulus-specific, or the interaction may be ‘amodal’ in the sense that it happens between the behavioural tendencies or ‘values’ which the respective stimuli have elicited. Here, we consider only five examples for these two kinds of interaction between visual and olfactory modalities in insects.
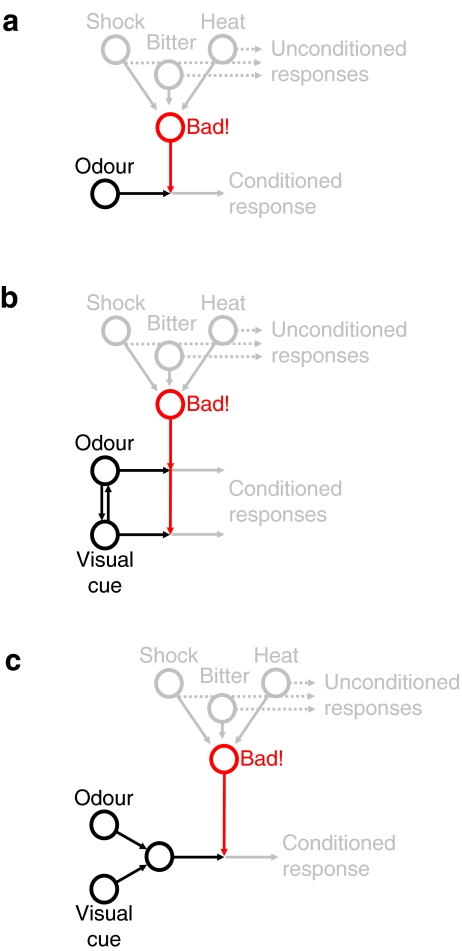
As an example of what we here call an ‘amodal’ interaction, consider the case of odour-shock learning: At the site of convergence, information about the particular features of the odour is maintained (i.e. in terms of the pattern of activated mushroom body neurons; Wang et al. 2004), but information about the particular features of the shock is not. That is, in addition to the reflex responses it elicits, shock induces a reinforcement signal carried by very few dopaminergic neurons impinging onto the mushroom bodies; these neurons most likely can be activated by any negative stimulus (in fruit flies: Schwaerzel et al. 2003; Riemensperger et al. 2005; Schroll et al. 2006; in honeybees: Vergoz et al. 2007; in crickets: Unoki et al. 2005; comparably, in monkeys, dopaminergic neurons carry a reward signal; Schultz et al. 1997). In other words, they act as a ‘funnel’ for different kinds of negative stimuli, conveying a general ‘Bad!’ signal. Thus, the actual interaction is between olfactory processing and an ‘amodal’ value signal (Fig. 3a).
Fig. 3.
‘Amodal’ versus ‘true’ interactions between sensory modalities. Processing of different sensory modalities may interact ‘truly’, that is, in a stimulus-specific manner, or the interaction may be ‘amodal’, that is, between the ‘values’ elicited by the respective stimuli rather than the actual stimulus features. a Odour-shock learning exemplifies an ‘amodal’ interaction: Shock and probably all other aversive stimuli feed into a common ‘Bad!’ signal, which interacts with the particular stimulus features of the odour. That is, the odour is associated with ‘something Bad!’ and thus will subsequently be avoided. b Sensory pre-conditioning on the other hand requires a ‘true’ interaction between sensory modalities: Initially, an odour and a visual cue are presented simultaneously in the absence of any reinforcer. This joint presentation endows both stimuli with the ability to ‘call up’ each other in a stimulus-specific manner. When in a subsequent experimental phase, one of the two is paired with aversive heat, the other is ‘called up’ as well and is also associated with the ‘Bad!’ signal. c Such stimulus-specific interaction is also required for biconditional discrimination, that is, learning about the combinations of odours and visual cues: Representations of both the odour and the visual cue must converge to form an additional joint representation of the two. This joint representation then can be associated with the ‘Bad!’ signal
A similar interaction takes place when two stimuli relate to a common reinforcer: Honeybees learn odours as predictors for sugar more readily when these odours are accompanied by visual cues, which, in a first experimental phase, had already been learned to predict sugar (Gerber and Smith 1998). Likewise, in adult fruit flies, aversive olfactory learning during tethered flight is facilitated specifically by already learnt visual cues (Guo and Guo 2005). In both cases, it does not matter which particular visual stimulus is present as long as it is a previously learnt one. Therefore, also in these cases, the actual interaction is between olfactory processing and a value signal—specifically, the value signal triggered by the learnt visual stimulus.
As an example of a ‘true’ interaction, consider the association of two cues with each other such that the occurrence of one ‘reminds’ of the other. Guo and Guo (2005) exposed adult fruit flies, during tethered flight, simultaneously to an odour and a visual cue without any reinforcement. Then, in a second experimental phase, they trained the flies in the absence of the visual cue such that flying towards the odour resulted in heat punishment. After such training, flies not only avoided the punishment-associated odour but interestingly also that particular visual cue which had previously been associated with the odour, but which itself was never associated with heat (sensory preconditioning). This suggests that the odour and the visual cue may be able to specifically ‘call up’ each other by virtue of their initial joint presentation; in other words, such joint presentation must endow, for example, the odour with the capacity to trigger a functional visual representation despite the actual absence of the visual stimulus (Fig. 3b). The neuronal circuitry to accomplish such a task remains to be identified.
A ‘true’ stimulus-specific interaction is also required for biconditional discrimination learning. In such a task, one odour is reinforced in light, but not in darkness; whereas another odour is reinforced in darkness, but not in light (see sketches in Fig. 2). Thus, neither the odours nor the visual situation alone can reliably predict reinforcement, but only the combination of both can. Crickets (Matsumoto and Mizunami 2004) as well as cockroaches (Sato et al. 2006) readily master such kind of task. On the other hand, neither in adult (this study; Fig. 2) nor in larval (Yarali et al. 2006) fruit flies any evidence for biconditional discrimination learning across sensory modalities has so far been found. This kind of learning clearly requires a combinatorial stage of olfactory and visual processing (Rudy and Sutherland 1992); in other words, there must be a stage of processing where olfaction and vision converge such that a combined signal can enter into association with reinforcement (thus, different from the situation concerning sensory preconditioning, the interaction must be downstream of the initial sensory representation; Fig. 3c). Indeed, in honeybees (Mobbs 1982; Ehmer and Gronenberg 2002), cockroaches (Strausfeld and Li 1999) and crickets (Honegger and Schurmann 1975), afferents from antennal lobes and optic lobes converge onto the mushroom bodies, whereas in Drosophila, direct visual input to mushroom bodies is not evident (Otsuna and Ito 2006). Thus, there appears a correspondence between the availability of visual input to the mushroom bodies and the ability for biconditional discrimination across the visual and olfactory modality.
In any event, the enhancing effect of light on olfactory learning and recall (Fig. 1) stands apart from both kinds of interaction discussed so far. We find that olfactory memory is recalled independent of whether the present visual context matches between training and test. Thus, neither an ‘amodal’ value signal nor any feature of the visual context seems to be integrated with olfactory memory. Instead, visual context may influence olfactory learning and recall indirectly, for example via altering motor activity, alertness or olfactory acuity. This kind of effect would not require a specific interaction between olfactory and visual circuits.
In summary, there does not seem to be a general rule concerning the organisation of insect behaviour across sensory modalities. Rather, whether and exactly which kinds of cross-modality interaction is found seems to depend on the particular requirements of the behavioural task and the evolutionary preparedness, that is, the available circuitry of the particular species to handle it.
Acknowledgements
Supported by the Boehringer Ingelheim Fonds (PhD fellowship to A.Y.) and the German-Israel Foundation for Scientific Research and Development (GIF 1326-202.8/ 2003, to B.G.). Special thanks to E. Münch for financial support to A.Y. Experiments reported here comply with applicable law. The continuous support of the members of the Würzburg group, especially of M. Heisenberg, K. Oechsener and H. Kaderschabek, is gratefully acknowledged. Many thanks to R. Menzel (Freie Universität Berlin) for critical discussions.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Ayse Yarali, Phone: +49-931-8884483, FAX: +49-931-8884452, Email: ayse.yarali@biozentrum.uni-wuerzburg.de.
Bertram Gerber, Phone: +49-931-8884483, FAX: +49-931-8884452, Email: bertram.gerber@biozentrum.uni-wuerzburg.de.
References
- Ehmer B, Gronenberg W. Segregation of visual input to the mushroom bodies in the honeybee (Apis mellifera) J Comp Neurol. 2002;451:362–373. doi: 10.1002/cne.10355. [DOI] [PubMed] [Google Scholar]
- Fauria K, Dale K, Colborn M, Collett TS. Learning speed and contextual isolation in bumblebees. J Exp Biol. 2002;205:1009–1018. doi: 10.1242/jeb.205.7.1009. [DOI] [PubMed] [Google Scholar]
- Gerber B, Smith BH. Visual modulation of olfactory learning in honeybees. J Exp Biol. 1998;201:2213–2217. doi: 10.1242/jeb.201.14.2213. [DOI] [PubMed] [Google Scholar]
- Gerber B, Tanimoto H, Heisenberg M. An engram found? Evaluating the evidence from fruit flies. Curr Opin Neurobiol. 2004;14:737–744. doi: 10.1016/j.conb.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Guo J, Guo A. Crossmodal interactions between olfactory and visual learning in Drosophila. Science. 2005;309:307–310. doi: 10.1126/science.1111280. [DOI] [PubMed] [Google Scholar]
- Heisenberg M. Genetic approach to learning and memory (mnemogenetics) in Drosophila melanogaster. In: Lindauer M, editor. Fortschritte der Zoologie. Fundamentals of memory formation: neuronal plasticity and brain function. Stuttgart, Germany: G. Fischer; 1989. pp. 3–45. [Google Scholar]
- Honegger HW, Schurmann FW. Cobalt sulphide staining of optic fibres in the brain of the cricket, Gryllus campestris. Cell Tissue Res. 1975;159:213–225. doi: 10.1007/BF00219157. [DOI] [PubMed] [Google Scholar]
- Hvorecny LM, Grudowski JL, Blakeslee CJ, Simmons TL, Roy PR, Brooks JA, Hanner RM, Beigel ME, Karson MA, Nichols RH, Holm JB, Boal JG. Octopuses (Octopus bimaculoides) and cuttlefishes (Sepia pharaonis, S. officinalis) can conditionally discriminate. Anim Cogn. 2007;10:449–459. doi: 10.1007/s10071-007-0085-4. [DOI] [PubMed] [Google Scholar]
- Liu G, Seiler H, Wen A, Zars T, Ito K, Wolf R, Heisenberg M, Liu L. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439:551–556. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Mizunami M. Olfactory learning in the cricket Gryllus bimaculatus. J Exp Biol. 2000;203:2581–2588. doi: 10.1242/jeb.203.17.2581. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Mizunami M. Context-dependent olfactory learning in an insect. Learn Mem. 2004;11:288–293. doi: 10.1101/lm.72504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs PG. The brain of the honeybee Apis mellifera. I. The connections and spatial organization of the mushroom bodies. Philos Trans R Soc Lond B. 1982;298:309–354. doi: 10.1098/rstb.1982.0086. [DOI] [Google Scholar]
- Otsuna H, Ito K. Systematic analysis of the visual projection neurons of Drosophila melanogaster. I. Lobula-specific pathways. J Comp Neurol. 2006;497:928–958. doi: 10.1002/cne.21015. [DOI] [PubMed] [Google Scholar]
- Riemensperger T, Voller T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Sutherland RJ. Configural and elemental associations and the memory coherence problem. J Cogn Neurosci. 1992;4:208–216. doi: 10.1162/jocn.1992.4.3.208. [DOI] [PubMed] [Google Scholar]
- Sato C, Matsumoto Y, Sakura M, Mizunami M. Contextual olfactory learning in cockroaches. Neuroreport. 2006;17:553–557. doi: 10.1097/01.wnr.0000209002.17610.79. [DOI] [PubMed] [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ, Li Y. Organization of olfactory and multimodal afferent neurons supplying the calyx and pedunculus of the cockroach mushroom bodies. J Comp Neurol. 1999;409:603–625. doi: 10.1002/(SICI)1096-9861(19990712)409:4<603::AID-CNE7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Unoki S, Matsumoto Y, Mizunami M. Participation of octopaminergic reward system and dopaminergic punishment system in insect olfactory learning revealed by pharmacological study. Eur J Neurosci. 2005;22:1409–1416. doi: 10.1111/j.1460-9568.2005.04318.x. [DOI] [PubMed] [Google Scholar]
- Vergoz V, Roussel E, Sandoz JC, Giurfa M. Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PLoS ONE. 2007;2:e288. doi: 10.1371/journal.pone.0000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo HF, Pologruto TA, Hannan F, Hakker I, Svoboda K, Zhong Y. Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J Neurosci. 2004;24:6507–6514. doi: 10.1523/JNEUROSCI.3727-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R, Heisenberg M. Basic organization of operant behavior as revealed in Drosophila flight orientation. J Comp Physiol [A] 1991;169:699–705. doi: 10.1007/BF00194898. [DOI] [PubMed] [Google Scholar]
- Yarali A, Hendel T, Gerber B. Olfactory learning and behaviour are ‘insulated’ against visual processing in larval Drosophila. J Comp Physiol [A] 2006;192:1133–1145. doi: 10.1007/s00359-006-0140-7. [DOI] [PubMed] [Google Scholar]