Figure 8. Lactate-induced stabilization of HIF-1 α in oxygenated endothelial cells.
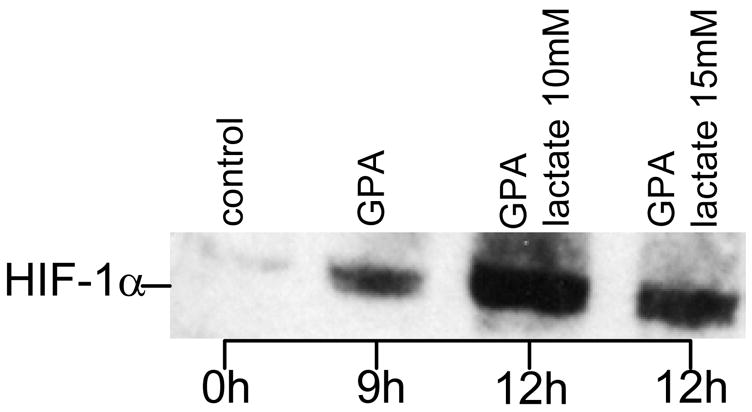
. Endothelial cells were grown in 6 well plates to sub-confluent monolayers with a cell density of approximately 106 cells/well. After overnight serum starvation, cells were treated for nine hours with the prolyl-hydroxylase inhibitor GPA1734 (8,9-dihydroxy-7-methyl-benzo[b]quinolizinium bromide; kindly provided by Dr. M. Maragoudakis, Patras, Greece) and HIF-1α protein was detected by Western blotting. For immunoblotting, cells were washed with ice-cold PBS and collected by scraping into 1 ml PBS. After centrifugation at 1000 g for 5 min, pellets were homogenized with an equal volume of lysis buffer (20 mM Tris-HCl, pH 7.5 containing 150 mM NaCl) (Cell Signaling, Beverly, MA) containing protease inhibitors and incubated for 20 min on ice. After sonication for 15 seconds samples were subjected to centrifugation at 15,000 g for 20 min at 4 ° to separate membrane and cytosolic fractions, suspended in 2X SDS sample buffer (Cell Signaling, Beverly, MA) containing 1 mM DTT, and boiled for 5 minutes. Equal quantities of protein were separated by SDS-PAGE electrophoresis under reducing conditions using a 7.5% Tris-HCl gel (Bio-Rad, Hercules, CA). After transfer onto 0.45 μm nitrocellulose membranes (Millipore, Marlborough, MA), the membranes were blocked with 5% non-fat dry milk and probed with anti HIF-1α (1:1500; Novus Biologicals, Littleton, CO). GPA 1734 was used to block the proteosomal degradation pathway of HIF-1α.
