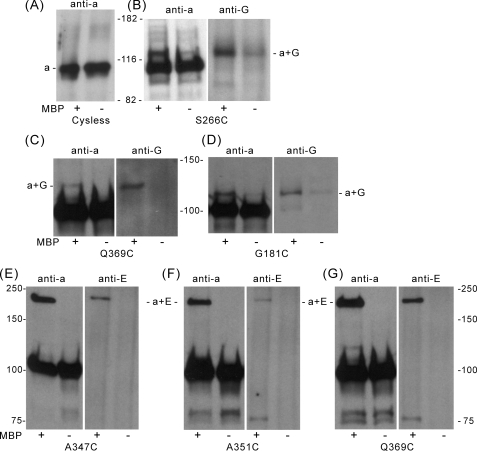
FIGURE 7.
The N-terminal domain of subunit a (Vph1p) can be cross-linked to subunits E and G of V1 using the photoactivated cross-linking reagent MBP. Vacuolar membrane vesicles isolated from yeast expressing the Cys-less form of subunit a (Vph1p) or the indicated single cysteine-containing forms of Vph1p were incubated in the presence (+) or absence (–) of 1 mm MBP for 30 min at room temperature. Excess MBP was quenched by addition of 10 mm dithiothreitol and removed by washing the samples with phosphate-buffered saline-EDTA buffer by sedimentation. After resuspension, the samples were irradiated with a long wavelength ultraviolet lamp (366 nm) for 5 min at 4 °C, solubilized with 2% C12E9 and immunoprecipitated with an antibody against subunit A to precipitate intact complexes. The immunopurified proteins were separated by SDS-PAGE using a 7.5% Pre-Cast Gel and transferred to nitrocellulose membranes. Western blot analysis was then performed using an antibody against subunit a (Vph1p) or other subunit-specific antibodies, as indicated. A, vacuolar membranes from yeast expressing the Cys-less subunit a (Vph1p); staining performed with the anti-Vph1p antibody, indicating the absence of higher molecular weight cross-linked products in the membranes containing the Cys-less form of Vph1p. B–D, vacuolar membranes from the S266C, Q369C, and G181C mutants; staining performed with antibodies against subunits a and G, showing the MBP-dependent formation of a cross-linked product of molecular mass 110–130 kDa recognized by antibodies against both subunits a (Vph1p) and G. E–G, vacuolar membranes from the A347C, A351C, and Q369C mutants; staining performed using antibodies against subunits a and E, showing the MBP-dependent formation of a cross-linked product of molecular mass of ∼200 kDa recognized by antibodies against both subunits a (Vph1p) and E.