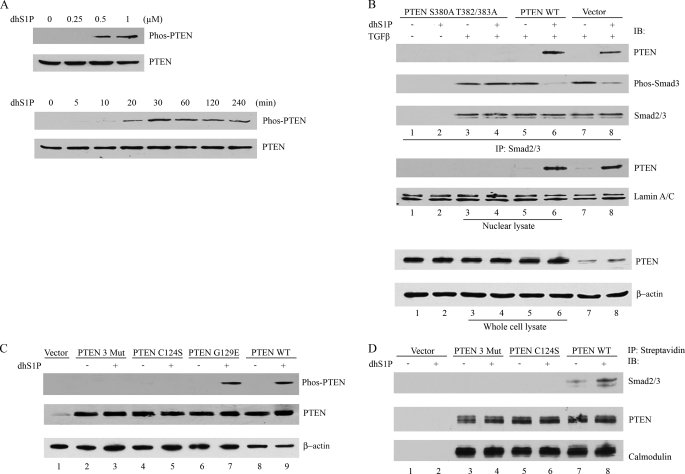
FIGURE 5.
C-terminal phosphorylation of PTEN mediates dhS1P-induced PTEN nuclear translocation and dephosphorylation of Smad3. A, dhS1P stimulates C-terminal phosphorylation of PTEN in a dose-dependent (top) and time-dependent (bottom) manner. C-terminal phosphorylation of PTEN was detected by immunoblotting. B, HEK 293 cells were transfected with the phosphorylation-deficient PTEN mutant, PTENWT, or vector only for 24 h and then incubated with 0.5 μm dhS1P and 2.5 ng/ml TGF-β for 30 min. The nuclear fraction was immunoprecipitated (IP) by Smad2/3 and then probed for PTEN, phospho-Smad3, and Smad2/3 by Western blot (IB). PTEN was also tested in nuclear lysate and whole cell lysate. Lamin A/C and β-actin served as controls for nuclear and cytoplasmic fractions, respectively. C, HEK 293 cells were transfected with the phosphorylation-deficient PTEN mutant (Mut3), PTEN C124S, PTEN G129E, and PTENWT and incubated with 0.5 μm dhS1P for 30 min. C-terminal phosphorylation of PTEN was detected by Western blotting. D, C-terminal phosphorylation (Mut3) and C124S PTEN mutants do not associate with Smad2/3. The PTEN proteins contained calmodulin and streptavidin tags from the pCTAP vector (Stratagene). Streptavidin-coupled agarose beads were used to pull down wild-type and mutated PTEN. Smad2/3 and PTEN were detected by immunoblotting. Immunoblotting with calmodulin served as control for equal loading.