Abstract
Objective
To characterize the integrity of non-nutritive suck (NNS) parameters among three groups of preterm infants ranging from normal to those with progressive degrees of respiratory distress syndrome (RDS).
Study Design
NNS compression waveforms were sampled from 55 infants in the neonatal intensive care unit using a silicone pacifier electronically instrumented for intraluminal pressure. Seven select NNS parameters were measured at two different sessions, and statistically analyzed using a General Linear Model Analysis of Covariance.
Results and Conclusions
Preterm infants with a more extensive history of RDS and oxygen therapy manifest significantly (p≤0.001) degraded performance on six of the seven NNS measures. This trend was disproportionately amplified in preterm infants with moderate-to-severe RDS. Prolonged periods of RDS requiring oxygen therapy may cause maladaptive orosensory experiences, and restrict oral movements which may contribute to delayed NNS development.
Keywords: Preterm birth, Non-nutritive suck, Respiratory distress syndrome, Sensory deprivation
Introduction
The ontogeny of suck is marked by rhythmic bursts of fetal non-nutritive suck (NNS) activity and occurs at approximately 28-33 weeks gestational age (GA, i.e. the time elapsed between the first day of the last menstrual period and the day the infant is born) (Hack et al., 1985). The nutritive suck differs from NNS in that the expression of milk requires simultaneous coordination of suck, swallow and respiration (Medoff-Cooper, 2005), with slower suck cycles (1 Hz) and generally without inter-burst pauses (Wolff, 1968). Coordinated NNS is an important precursor to successful oral feeding and demonstrates remarkable stability by 34 weeks GA (Hack et al., 1985).
Infants who are born prematurely frequently exhibit oromotor dysfunction and are unable to suck and feed orally which can lead to an extended stay in the neonatal intensive care unit (NICU). An underdeveloped nervous system compounded by the necessity to face the environmental demands of extrauterine life presents a significant challenge to the formation of neural circuits which support adaptive sensorimotor control. The suck central pattern generator (sCPG) is one such highly adaptive neuromotor behavior that appears susceptible to prematurity. The sCPG is regulated by a bilateral neural network of pontine interneurons in the human infant which can be modulated by central descending inputs (sensorimotor cortex) and peripheral somatosensory inputs (Barlow & Estep, 2006).
Unstable neonatal conditions associated with lung disease or respiratory distress syndrome (RDS) require oxygen supplementation which means trussing the lower face with tubes and tape (Case-Smith, 1989). Unfortunately, these procedures produce unexpected tactile stimulation and movement restriction of the lower face that may negatively affect the development of the sCPG (Comrie & Helm, 1997; Finan & Barlow, 1996). In animal models, the combination of sensory deprivation and motor restriction has been shown to disrupt development of key brain structures involved in sensorimotor control, including motor cortex and cerebellum (Pascual & Figueroa, 1996; Pascual et al., 1993; Pascual et al., 1998). This is consistent with the notion of a critical period during early postnatal life, when manipulations in trigeminal sensory systems may significantly alter the structure and function of the developing brain (Bosma, 1973). Somatosensory information associated with oromotor behaviors is crucial in order for infants to integrate sensorimotor experiences in their environment, and such atypical oromotor experiences may significantly disturb the development of NNS and delay the transition to competent oral feeds.
Little is known about the motor dynamics of infant ororhythmic development, nor its response to rate limiting conditions such as the oral sensorimotor deprivation associated with RDS. Therefore, the purpose of this study was to characterize salient NNS parameters among three preterm groups, including healthy CONTROL (minimal oxygen history), RDS1 (mild oxygen history), and RDS2 (moderate-severe oxygen history) babies. We hypothesized the integrity of the NNS would be degraded as a function of RDS severity.
Patients & Methods
Patients
This study was approved by the human subjects committees of the University of Kansas Medical Center (Kansas City, KS) and Stormont-Vail Regional Medical Center (Topeka, KS) and written informed consent was obtained from the parents or guardians of the infants prior to study participation. Participants were 55 preterm infants (23 female, 32 male), with a mean GA of 30.06 weeks (SD 2.2) and mean birth weight of 1339.7 grams (SD 386.2). These infants were distributed among three groups based on oxygen supplementation history: CONTROL (no intubation, minimal or no oxygen history, range 0-4 days), RDS1 (Respiratory Distress Syndrome, mild oxygen history requiring endotracheal intubation and/or 5-7 days of oxygen therapy), and RDS2 (Respiratory Distress Syndrome, moderate-to-severe oxygen history requiring endotracheal intubation and more than 7 days of oxygen therapy). The clinical characteristics of each group are given in Table I.
Table I. Clinical Characteristics of Study Infants*.
| VARIABLE | CONTROL
(n=17) |
RDS1
(n=11) |
RDS2
(n=27) |
|
|---|---|---|---|---|
| Gender (males : females) | 8 : 9 | 7 : 4 | 17 : 10 | |
| Birth GA (weeks) | 31.5 (1.4) | 30.5 (2.1) | 29.0 (2.2) | |
| Birth Weight (grams) | 1518.7 (318.6) | 1442.4 (275.1) | 1185.3 (409.9) | |
| PMA @ Session (weeks) | Session 1 | 33.6 (1.7) | 33.4 (1.3) | 34.1 (2.2) |
| Session 2 | 34.7 (1.6) | 34.5 (1.1) | 34.9 (2.1) | |
| Mean | 34.2 (1.7) | 34.0 (1.2) | 34.5 (2.2) | |
| % Oral Feeding | Session 1 | 13.2 (4.0) | 9.4 (4.4) | 3.4 (3.2) |
| Session 2 | 35.0 (9.5) | 40.6 (10.5) | 10.4 (7.5) | |
| Mean | 24.1 (6.8) | 25.0 (7.5) | 6.9 (5.4) | |
| Oxygen Therapy History (days) | Ventilator | 0.0 (0.0) | 1.3 (1.3) | 6.4 (11.0) |
| CPAP | 0.7 (1.1) | 2.3 (2.2) | 9.6 (10.2) | |
| Cannula | 0.7 (1.3) | 1.6 (1.6) | 21.9 (15.2) | |
| Total | 1.4 (1.7) | 5.2 (1.8) | 37.9 (26.0) | |
Expressed as mean (sd)
Inclusion criteria for the study population were: head circumference within 10-90th percentile of mean for post-menstrual age (PMA, i.e. the time elapsed between the first day of the last menstrual period plus the time elapsed after birth), neurological examination showing no anomalies for PMA: response to light, sound, spontaneous movements of all extremities, and stable vital signs (heart rate, blood pressure, age appropriate respiratory rate, and oxygen saturation >92 SpO2) to allow for NNS. All infants were extubated for >5 days at the time of testing. Exclusion criteria were: all grades of intracranial hemorrhage, periventricular leukomalacia, neonatal seizures and culture positive sepsis or meningitis at time of testing, chromosomal anomalies or craniofacial malformation.
Equipment and Data Collection
Infants were tested at the neonatal intensive care units over two sessions occurring approximately one week apart (mean = 6.8 days apart [SD = 2.5]), starting at 34 weeks PMA. Fifteen minutes before feeding, the mobile Actifier (Finan & Barlow, 1996) recording station, designed and developed in our laboratory, was positioned cribside. The Actifier was used to sample NNS compression waveforms with a sterile Soothie™ silicone pacifier (Children's Medical Ventures, Inc.) snapped onto a specially designed receiver, which included a lubricated spherical acetal head and stainless steel cannula with a Luer fitting coupled to a Honeywell pressure transducer. The Actifier was controlled by Neosuck RT©, a specialized software program developed in our laboratory for use in the NICU which communicates with the National Instruments PCI-6052E real time multifunction data acquisition card. Outputs from the Actifier's integrated pressure sensor were conditioned by a bridge amplifier (DC-coupled, Butterworth 3-pole LP filter @ 50 Hz), and digitized (3 kHz, 16 bits vertical resolution) in real time. A 2-point scale calibration of intraluminal air pressure for the Soothie™ pacifier was completed using water manometry and registered with each data file prior to digitization.
Following a brief examination of physiologic state, the infant was cradled in a supportive inclined posture, swaddled, with limbs positioned at midline, and background/overhead lighting dimmed in the isolette suites to promote eye contact with the tester. Sampling of NNS behavior was not initiated until the infant was in an optimal behavioral state, i.e., drowsy to quiet alert (stages 3 or 4 of the Preterm Infants Behavioral Scale, Newborn Individualized Developmental Care and Assessment Program; NIDCAP) (Als, 1995). The infant was presented the instrumented silicone pacifier to establish an oral latch on the nipple, and several minutes of NNS data were sampled. The infant remained connected to the NICU life support monitors at all times for observation of respiration, heartbeat and oxygen saturation. Physiological parameters of oxygen therapy requirement, PMA, and nurse's report of oral feeding ability were documented.
Data Analysis
Two minutes of continuous data were chosen based on the greatest number of pressure peaks, reflecting each infant's most active period of oromotor output. A specialized peak picking algorithm implemented in NeoSuck RT© utilized the first derivative of pressure and threshold intercepts to tag individual pressure cycles of a NNS burst. Peak pressure intercepts for time and amplitude were obtained by indexing each zero crossing of the derivative suck signal into the original suck signal data. This algorithm permitted objective identification of NNS activity occurring within the two-minute data sample associated with a single test session.
Seven dependent variables were quantitatively analyzed for each two-minute data sample. Waveform discrimination and threshold detection set to 1 cm H2O was used for objective identification of nipple compression events. The minute-rate variables included: (1) Total Mouthing Events defined as the sum of all pressure events, (2) Non-NNS Events defined as nipple compression pressure events that did not occur within an NNS burst sequence, (3) Burst-related NNS Cycles defined as suck compression cycles with cycle periods less than 1000 milliseconds and occurring within the NNS burst structure, and (4) NNS Bursts defined as the cumulative number of NNS bursts produced per minute. NNS bursts consisted of two or more compression cycles. The three remaining global measures included the (5) Average Number of NNS Cycles/Burst, and two ratiometric calculations which highlighted the proportions of organized ororhythmic activity produced among the preterm infant test groups, including (6) NNS Cycles as a percentage of the Total Number of Mouthing Events (Burst-related NNS cycles/Total Mouthing Events ∗ 100), and its complement component, namely (7) Non-NNS Events as a percentage of the Total Number of Mouthing Events (Non-NNS Events/Total Mouthing Events ∗ 100).
Statistical Analysis
A General Linear Model Analysis of Covariance (ANCOVA) was completed for each NNS variable measured at two different sessions. The purpose of the ANCOVA is to increase the sensitivity of the test of main and interaction effects by reducing the error variance. Since the NNS suck variables were measured twice at a one week interval there is a between-subject factor (i.e., mixed design). The major question for ANCOVA in the current study is whether or not there are group, measurement, and/or group by measurement differences in the mean NNS suck variable scores after they are adjusted for differences in the covariate scores. Thus, the between-subjects factor was preterm group (CONTROL, RDS1, and RDS2) and the repeated-measures factor was weekly session (session 1 and 2). Covariates included GA at birth, PMA at the first session, and birth weight. The mean differences were considered significant if p<0.05, and the Bonferroni adjustment was used for multiple pairwise comparisons. Analyses were performed using SPSS v.15.
Results
An evaluation of assumptions of normality, linearity, homogeneity of variance-covariance matrices, multicollinearity, singularity, and sphericity were satisfactory. Repeated measures ANCOVA revealed a significant main effect of group (p≤0.001) for six of the seven dependent variables (Table II). The effects size, Eta2, ranged from 0.32 to 0.62 indicative of medium to large effects for the significant suck variables. Post hoc tests indicated the RDS2 group had significantly reduced oromotor output compared to the CONTROL and RDS1 groups on NNS variables. Although the minute-rate of non-NNS events did not significantly differ between groups, the proportion of non-NNS events of the total mouthing events was significantly greater in the RDS2 group compared to the CONTROL and RDS1 groups (Table III). This indicates differences in the minute-rate of total mouthing events between groups are primarily driven by the quantity of organized, burst-related NNS cycle activity. The absence of any significant interactions between session and group indicated the patterns observed in each oromotor variable were consistent between the two weekly sessions.
Table II. ANCOVA Results.
| Oromotor Variable | df | F | Sig | Eta2 |
|---|---|---|---|---|
| Total Mouthing Events/min | 2, 49 | 12.270 | 0.000 | 0.50 |
|
| ||||
| Non-NNS Events/min | 2, 49 | 2.018 | 0.144 | 0.08 |
| Burst Related NNS Cycles/min | 2, 49 | 15.303 | 0.000 | 0.62 |
| NNS Bursts/min | 2, 49 | 9.527 | 0.000 | 0.39 |
| Average Number of NNS Cycles/Burst | 2, 49 | 7.952 | 0.001 | 0.32 |
| NNS Cycle % of Total Mouthing Events | 2, 49 | 12.166 | 0.000 | 0.50 |
| Non-NNS Cycle % of Total Mouthing Events | 2, 49 | 12.166 | 0.000 | 0.50 |
Table III. Outcome Variables.
| Oromotor Variable | CONTROL | RDS1 | RDS2 |
|---|---|---|---|
| Total Mouthing Events/min | 41.4 (2.9) | 44.4 (3.2) | 25.5 (2.3)** |
| Non-NNS Events/min | 5.3 (1.7) | 5.8 (1.9) | 9.7 (1.3) |
| Burst Related NNS Cycles/min | 36.1 (3.2) | 38.6 (3.5) | 15.8 (2.5)*** |
| NNS Bursts/min | 5.4 (0.5) | 5.6 (0.5) | 2.9 (0.4)* |
| Average Number of NNS Cycles/Burst | 7.2 (0.6) | 7.5 (0.7) | 4.4 (0.5) * |
| NNS Cycle % of Total Mouthing Events | 89.4 (6.2) | 86.3 (6.8) | 50.8 (4.9)** |
| Non-NNS Cycle % of Total Mouthing Events | 10.6 (6.2) | 13.7 (6.8) | 49.2 (4.9)** |
RDS2 differs significantly from CONTROL and RDS1 groups at the p =0.01*, p =0.001**, and p =0.0001*** alpha levels.
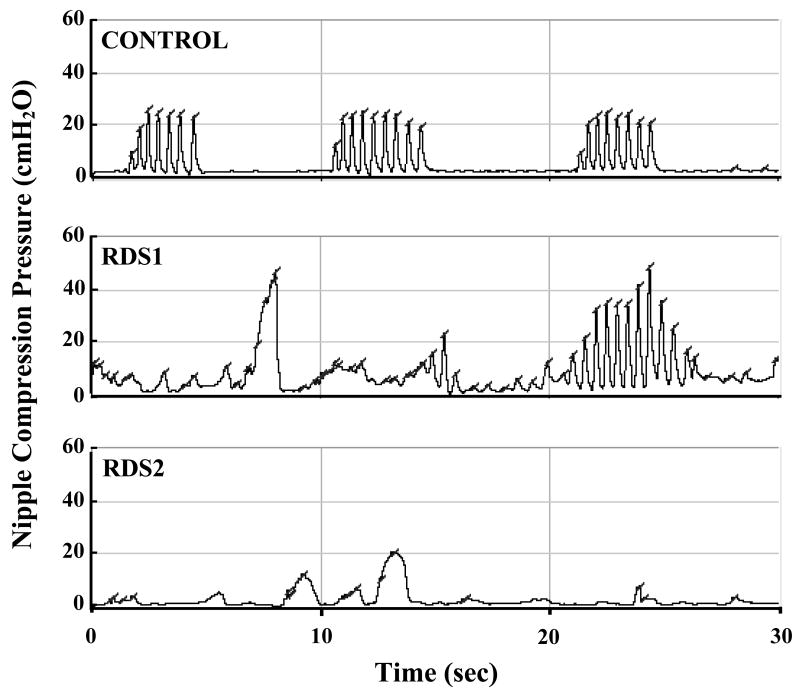
As shown in Figure 1, degradation of NNS structure appeared to follow a continuum with increasing severity of oxygen history from the CONTROL and RDS1 groups to the RDS2 group, with a disproportionate breakdown in oromotor control among RDS2 infants. The RDS2 group (average oxygen requirements: 37.4 days) endured longer periods of oxygen therapy compared to the CONTROL and RDS1 groups (average oxygen requirements: 1.4 days and 5.1 days, respectively). Of the three samples, the RDS2 group produced the highest non-NNS event percentage of total mouthing events (3.4 times greater than CONTROL), presumably indicating the output from a maladaptive neural circuitry underlying the sCPG. Such a lack of structured development highlighted by atypical oral movements clearly distinguished the RDS2 babies from the other groups.
Figure 1. Oromotor Sample Profiles.
Sample non-nutritive suck waveforms characteristic of healthy CONTROL, RDS1, and RDS2 infants. Three NNS bursts evident in CONTROL panel. One NNS burst and several non-NNS burst mouthings apparent in RDS1 record. No NNS burst structure in RDS2 panel.
Discussion
The potent nature of sensory input on development of motor systems is well documented in studies of cortical assembly. During early postnatal life, cortical layer 4 and thalamocortical pathways are very plastic allowing sensory-dependent development (Erzurumlu & Jhaveri, 1990). Cortical structure and function can be enhanced or impaired depending on characteristics of the activity in thalamocortical afferents (Miller et al., 1993). The extent of cortical reorganization points to the importance of correlated neural activity in the formation and maintenance of cortical representations of the body surface (Merzenich et al., 1984). Thus, sensory consequences associated with spontaneous and goal-directed movements provide the baby's brain with important cues for axonal guidance and synaptic reinforcement that are continually encoded, mapped in the brain, and encourage prompt development (Shatz, 1994). The status of the neural representation of orofacial systems is hypothesized to determine the behavioral output, and behavior itself changes the neuromotor system. Under these guiding principles, both spontaneous and patterned movements of the lips, tongue and jaw for suck, typically serve to provide the baby with a rich source of temporally salient somatosensory stimuli, which in turn reinforces the coordination of many muscle systems involved in producing the burst-pause pattern of NNS.
Feeding behavior in preterm infants matures significantly between 33-36 weeks PMA, and has been suggested to be predictive of future motor performance (Amiel-Tison & Gosselin, 2001). Preterm infants who show improvements in feeding patterns have been found to develop normally or with only minor neurodevelopmental delays at 18 months of age (Mizuno & Ueda, 2005). In comparison, premature infants who lack a functional suck or manifest oromotor dysfunction that persists well into early childhood are at significant risk for neurodevelopmental delay (Stoll et al., 2004). The lengthy intubation procedures cost the baby precious sensory and motor experiences during a critical period of brain development for oromotor pattern generation. The unusual orosensory environment resulting from the presence of the ventilator tube may influence perceptual-motor experience and interfere with movement pattern formation compared to non-intubated babies (Barnard & Bee, 1983; Bier et al., 1993). Bosma (1973) suggested that “appropriate oral experiences may be critical in the final weeks of gestation, and that their interruption may impair fragile syntheses of central neural representations of these functions.” The importance of experience in brain development and plasticity is highlighted by the resultant physiological and cortical structural changes that modify motor and sensory system circuitry (Pascual et al., 1993; Pascual et al., 1998). Infants with perinatal distress and neurologic impairment compared to normal full-term infants manifest a significantly slower mean rate of NNS and a greater intra-individual variability of rate (Dreier & Wolff, 1972). This supports the hypothesis that NNS is indeed sensitive to perinatal distress and rhythmic oromotor activities could be sensitive indicators of minor disturbances of central nervous system (CNS) function in infants without any other obvious neurologic impairment (Medoff-Cooper & Ray, 1995). The usual activity-dependent associations reinforced through correlated sensorimotor activity in neurally organized babies are presumed to be degraded in infants who manifest oromotor dysfunction, a potential marker for discoordination within the CNS (Medoff-Cooper et al., 1993; Wolff, 1968).
In summary, NNS performance varies significantly as a function of RDS severity. In our view, RDS infants represent a test model of oral sensory and motor deprivation, depending upon the type and duration of oxygen therapy. The reduced opportunities for oromotor experiences, such as suck or autostimulation, in this sample are hypothesized to result in a delay in the development of brain circuits responsible for organized suck. Quantitative measures of the intraluminal pressure during NNS are designed to provide the clinician with a rapid, non-invasive and objective means to assess the integrity of the sCPG in the preterm infant. Repeated measures in the NICU at weekly or even daily intervals may serve to guide therapeutic intervention and assess feeding readiness skills.
Acknowledgments
Supported in part by NIH R01 DC03311 (SM Barlow), NIH P30 HD02528, and NIH P30 DC005803. Special gratitude to Susan Cannon, MS, PT, at University of Kansas Medical Center, Joy Carlson, NNP, and Jose Gierbolini, MD, Director of the NICU at Stormont-Vail Regional Medical Center who cared for study patients. We would also like to thank Susan Stumm, Lana Seibel, Meredith Poore, Emily Zimmerman, Monique Fees, Shinying Chu, Mimi Urish and Kendra Gagnon for data collection.
Supported by: NIH R01 DC03311-06, NIH P30 HD02528, and NIH P30 DC005803
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Meredith Estep, Graduate Research Associate, Communication Neuroscience Laboratories, Program in Neuroscience, University of Kansas, Lawrence, Kansas USA.
Steven M. Barlow, Professor, SPLH, Programs in Neuroscience and Human Biology, Director Communication Neuroscience Laboratories, University of Kansas, Lawrence, Kansas USA.
Rajesh Vantipalli, Communication Neuroscience Laboratories, University of Kansas, Lawrence, Kansas USA.
Donald Finan, Assistant Professor, Department of Speech-Language-Hearing Science, Center for Neuroscience, University of Colorado, Boulder, Colorado USA.
Jaehoon Lee, Statistician, Advanced Statistical Methods Core, NIH Center for Biobehavioral Neurosciences in Communication Disorders, University of Kansas, Lawrence, Kansas USA.
References
- Als H. A manual for naturalistic observation of the newborn (preterm and full term infants) In: Goldson E, editor. Nurturing the premature infant, Developmental Interventions in the Neonatal Intensive Care Nursery. Oxford University Press; New York: 1995. pp. 77–85. [Google Scholar]
- Amiel-Tison C, Gosselin J. Neurological Development from Birth to Six Years: Guide for Examination and Evaluation. Johns Hopkins University Press; Baltimore: 2001. [Google Scholar]
- Barlow SM, Estep M. Central pattern generation and the motor infrastructure for suck, respiration, and speech. J Commun Disord. 2006;39:366–380. doi: 10.1016/j.jcomdis.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Barnard KE, Bee HL. The impact of temporally patterned stimulation on the development of preterm infants. Child Dev. 1983;54:1156–1167. [PubMed] [Google Scholar]
- Bier JA, Ferguson A, Cho C, Oh W, Vohr BR. The oral motor development of low-birth-weight infants who underwent orotracheal intubation during the neonatal period. Am J Dis Child. 1993;147:858–862. doi: 10.1001/archpedi.1993.02160320060020. [DOI] [PubMed] [Google Scholar]
- Bosma JF. Prologue to the symposium. In: Bosma JF, editor. Fourth Symposium on Oral Sensation and Perception. Charles C. Thomas; Bethesda: 1973. p. 7. [PubMed] [Google Scholar]
- Case-Smith J. Intervention strategies for promoting feeding skills in infants with sensory defects. Occup Ther Health Care. 1989;6:129–141. doi: 10.1080/J003v06n02_09. [DOI] [PubMed] [Google Scholar]
- Comrie JD, Helm JM. Common feeding problems in the intensive care nursery: maturation, organization, evaluation, and management strategies. Semin Speech Lang. 1997;18:239–261. doi: 10.1055/s-2008-1064075. [DOI] [PubMed] [Google Scholar]
- Dreier T, Wolff PH. Sucking, state, and perinatal distress in newborns. A preliminary report. Biol Neonate. 1972;21:16–24. doi: 10.1159/000240491. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Brain Res Dev Brain Res. 1990;56:229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- Finan DS, Barlow SM. The Actifier and neurophysiological studies of orofacial control in human infants. J Speech Hear Res. 1996;39:833–838. [PubMed] [Google Scholar]
- Hack M, Estabrook MM, Robertson SS. Development of sucking rhythm in preterm infants. Early Hum Dev. 1985;11:133–140. doi: 10.1016/0378-3782(85)90100-8. [DOI] [PubMed] [Google Scholar]
- Medoff-Cooper B. Nutritive sucking research: from clinical questions to research answers. J Perinat Neonat Nurs. 2005;19:265–272. doi: 10.1097/00005237-200507000-00013. [DOI] [PubMed] [Google Scholar]
- Medoff-Cooper B, Ray W. Neonatal sucking behaviors. J Nurs Sch. 1995;27:195–200. doi: 10.1111/j.1547-5069.1995.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Medoff-Cooper B, Verklan T, Carlson S. The development of sucking patterns and physiologic correlates in very-low-birth-weight infants. Nurs Res. 1993;42:100–105. [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, Cynader M, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Miller B, Chou L, Finlay BL. The early development of thalamocortical and corticothalamic projections. J Comp Neurol. 1993;335:16–41. doi: 10.1002/cne.903350103. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Ueda A. Neonatal feeding performance as a predictor of neurodevelopmental outcome at 18 months. Dev Med Child Neurol. 2005;47:299–304. doi: 10.1017/s0012162205000587. [DOI] [PubMed] [Google Scholar]
- Pascual R, Fernandez V, Ruiz S, Kuljis RO. Environmental deprivation delays the maturation of motor pyramids during the early postnatal period. Early Hum Dev. 1993;33:145–155. doi: 10.1016/0378-3782(93)90209-d. [DOI] [PubMed] [Google Scholar]
- Pascual R, Figueroa H. Effects of preweaning sensorimotor stimulation on behavioral and neuronal development in motor and visual cortex of the rat. Biol Neonate. 1996;69:399–404. doi: 10.1159/000244337. [DOI] [PubMed] [Google Scholar]
- Pascual R, Hervias MC, Toha ME, Valero A, Figueroa HR. Purkinje cell impairment induced by early movement restriction. Biol Neonate. 1998;73:47–51. doi: 10.1159/000013959. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. Role for spontaneous neural activity in the patterning of connections between retina and LGN during visual system development. Int J Dev Neurosci. 1994;12:531–546. doi: 10.1016/0736-5748(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- Wolff PH. The serial organization of sucking in the young infant. Pediatrics. 1968;42:943–956. [PubMed] [Google Scholar]