Fig. 4.
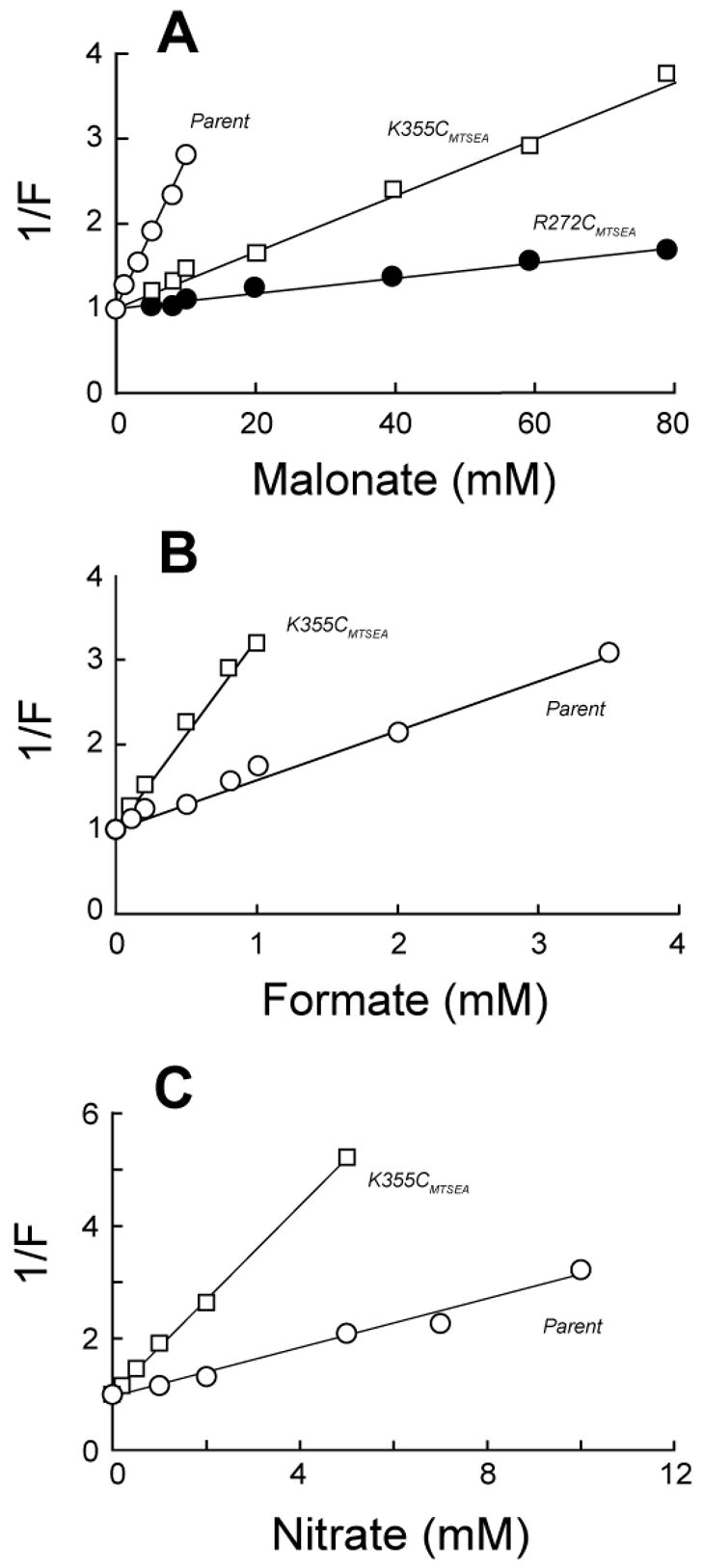
Altered substrate specificity for OxlT variants exposed to MTSEA. To determine apparent inhibition constants, the initial rates of 0.1 mM [14C]oxalate transport were determined in the presence of malonate (panel A), formate (panel B) or nitrate (panel C). Proteoliposomes treated with 1 mM MTSEA contained either cysteine-less OxlT (open circles), or its K355C (open squares) or R272C (closed circles) derivative. Observed rates were normalized by expressing them as a fraction (F) of the rate found in the absence of inhibitor, and apparent Ki values (Kiapp) were determined as the inhibitor concentrations yielding 50% inhibition of [14C]oxalate transport. Kiapp values cited in the text summarize three independent experiments as shown here. Assuming simple competitive inhibition and the kinetic parameters in Table I, the observed Kiapp values for malonate would reflect true Ki values of 72 mM, 53 mM, and 2.7 mM for R272CMTSEA, K355CMTSEA and cysteine-less OxlT, respectively; for formate, the respective Ki values would be 0.31 mM (K355CMTSEA) and 0.71 mM (cysteine-less OxlT); for nitrate, these Ki values would correspond to 0.7 mM (K355CMTSEA) and 1.8 mM (cysteine-less OxlT).
