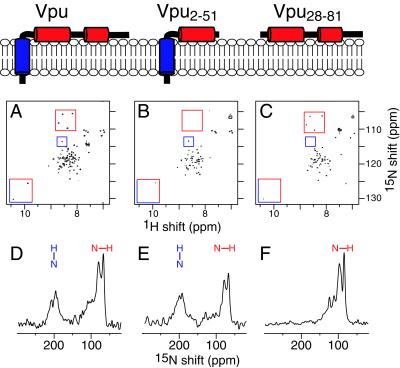
Figure 1.
NMR spectra of three uniformly 15N-labeled recombinant Vpu constructs. Vpu has three helical segments. (Top) The overall architecture of the three constructs used in this study in the context of a membrane bilayer, with the hydrophobic helix in blue and both amphipathic helices in red. (Middle) Two-dimensional heteronuclear single quantum correlation spectra of the three Vpu constructs in dihexanoyl phosphatidylcholine micelles: Vpu (A), Vpu2–51 (B), and Vpu28–81 (C). Representative assigned resonances are highlighted in the boxes: amide resonances from Gly-53, Gly-58, Gly-67, and Gly-71 (red boxes); Ser-23 (blue boxes); and indole resonances from Trp-22 and Trp-76 (red/blue boxes). (Bottom) One-dimensional solid-state 15N NMR spectra of the three Vpu constructs obtained at 0°C in oriented lipid bilayers: Vpu (D), Vpu2–51 (E), and Vpu28–81 (F). The orientations of transmembrane (blue) and in-plane (red) amide NH bonds are indicated above the spectra.