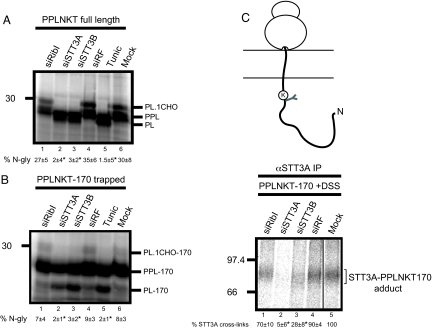
Fig. 3.
Molecular analysis of a ribophorin I−independent substrate. (A) Full-length PPLNKT was synthesized in vitro by using semipermeabilized HeLa cells prepared after siRNA mediated depletion of ribophorin I (lane 1), STT3A (lane 2), STT3B (lane 3), a nonfunctional control (siRF) (lane 4) or mock treatment (lane 6). Tunicamycin treatment served as a control (compare to Fig. 1). The resulting products, glycosylated (PL.CHO) and nonglycosylated (PL) prolactin and the precursor with intact signal sequence (PPL) are shown after SDS/PAGE. The relative proportion of glycosylated polypeptide was calculated for each sample and expressed as a percentage of the total protein recovered. The values below the lanes are the mean ± SEM of three independent experiments. Levels of N-glycosylation that differ from the mock treated control with a significance of P < 0.02 are indicated by asterisks. (B) A 170-residue PPLNKT translocation intermediate encoded by an mRNA lacking a stop codon was synthesized as before (compare to Fig. 2) and analyzed as described for A. (C) A proportion of the PPLNKT-170 translocation intermediates shown in B were treated with DSS and the resulting adducts with STT3A were recovered by immunoprecipitation. The amount of STT3A-PPLNKT-170 adduct for the mock sample was quantified and set to a nominal value of 100%. Other values are the mean of three independent experiments where the amount of adduct obtained after the different treatments is expressed relative to the mock treated sample. Values that differ from the mock treated control with a significance of P < 0.02 are indicated by asterisks.