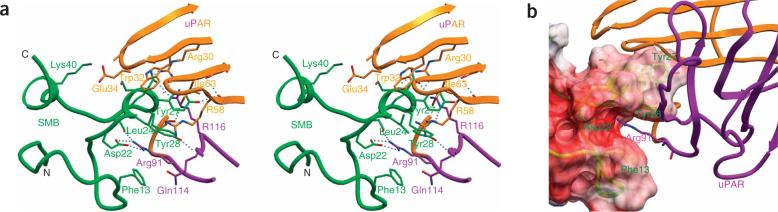
Figure 2.
Details of the uPAR–SMB interface. (a) Interaction of the vitronectin SMB domain with uPAR D1 (orange) and D2 domains (magenta) in stereoview. Selected contacting residues in ball-and-stick representation; hydrogen bonds are shown in dashed lines. (b) Residues Phe13, Tyr28 and Asp22 of vitronectin (in ribbon and transparent surface representation) form an open pocket to bind Arg91 of uPAR (in ribbon and stick). Tyr27 and Tyr28 of the SMB domain insert into a large cavity of uPAR, showing shape complementarity of this interface.